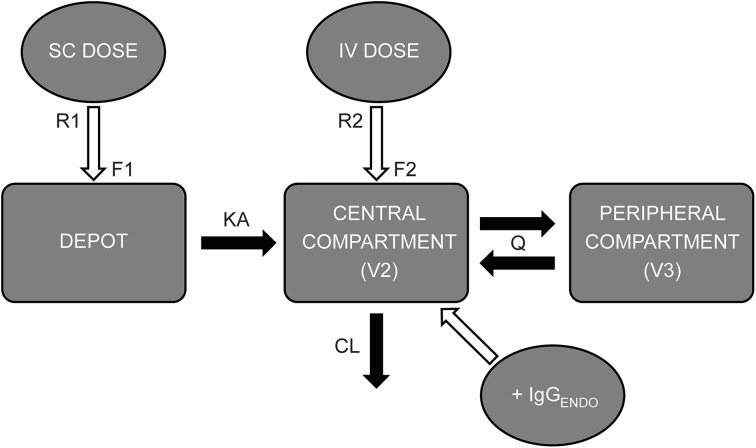
Fig. 1.
Schematic diagram of the two-compartment population PK model. CL clearance (L/day), F1 bioavailability of subcutaneous (SC) immunoglobulin, F2 bioavailability of intravenous (IV) immunoglobulin (=1.00), IgG ENDO endogenous serum IgG concentration (g/L), KA absorption rate constant of subcutaneous dose (day−1), Q inter-compartmental clearance (L/day), R1 rate of subcutaneous dose administration (g/day), R2 rate of intravenous administration (g/day), V2 volume of distribution of central compartment (L), V3 volume of distribution of peripheral compartment (L). Reprinted from Postgraduate Medicine, 125, Landersdorfer CB, Bexon M, Edelman J, et al. Pharmacokinetic modeling and simulation of biweekly subcutaneous immunoglobulin dosing in primary immunodeficiency, page 55, Copyright 2013, with permission from JTE Multimedia, LLC