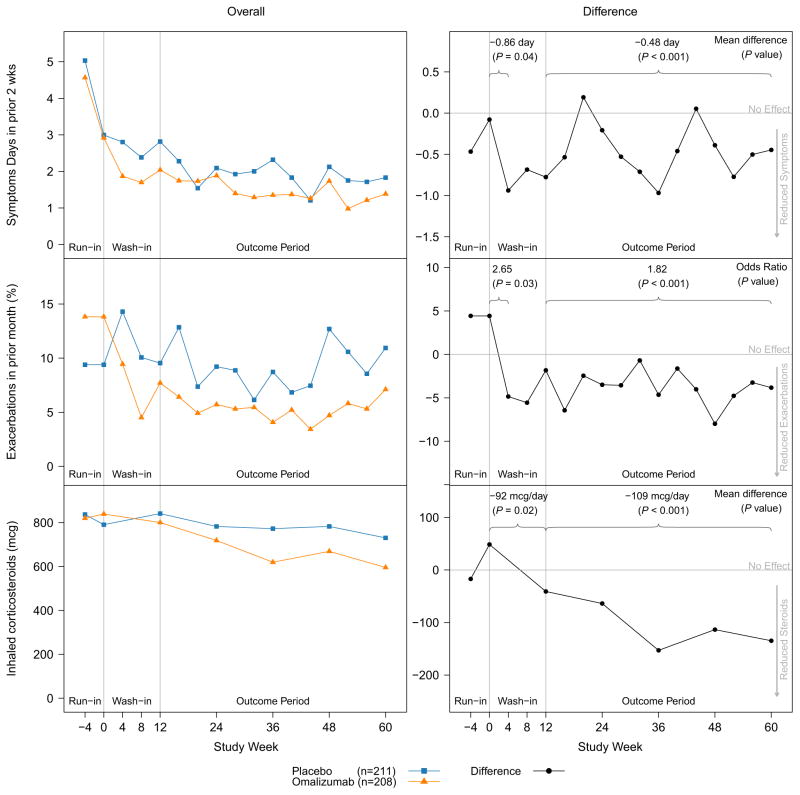
FIGURE 2.
Omalizumab time to effect for exacerbations and symptom days. Shown are changes in overall symptoms (top row), exacerbations (middle row), and ICS use (bottom row) between enrollment (week −4) and the end of the double-blind (week 60) in ICATA. Changes in group means (left column) are compared with effect size (right column) to better emphasize the timing of efficacy. The left column shows average effect sizes at the first symptom assessment (4 weeks for symptom days and exacerbations, 12 weeks for ICS) and over the course of the outcome period starting at week 12. During the outcome period, the relative improvement in symptoms was 24.5%, reduction in exacerbations was 37.9%, and reduction in ICS dose was 14.1%. From N Engl J Med. Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. Volume 364, pp. 1005–15. Copyright © 2011 Massachusetts Medical Society. Reprinted with permission.12