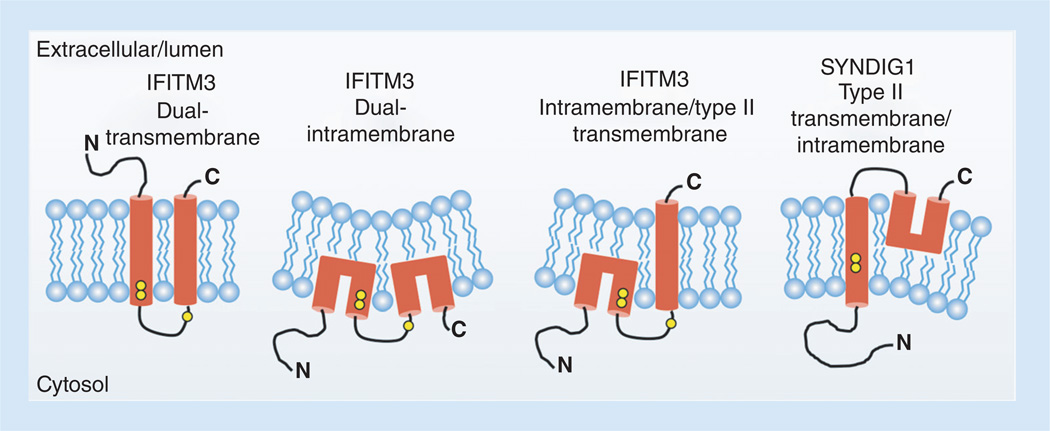
Figure 2. Membrane topologies for IFITM3 and SYNDIG1 that are supported by experimental evidence.
Data have been described supporting each of the shown topology models. Hydrophobic segments are shown in red. The IFITM3 dual transmembrane topology is predicted based on hydropathy plotting. However, IFITM3 in which its first hydrophobic segment adopts an intramembrane conformation is required for N-terminal access to cytoplasmic components necessary for its proper localization and activity. Whether the second hydrophobic segment in active IFITM3 traverses both membrane leaflets remains unclear. Yellow circles represent conserved cysteines that are known to be palmitoylated on IFITM3. Note that the cysteines in the first hydrophobic segment of SYNDIG1 appear earlier within the segment than IFITM3 cysteines making them more deeply embedded in the membrane and less likely to be palmitoylated. The third cysteine in SYNDIG1 is absent, agreeing with its experimentally determined topology. Potential changes in membrane properties induced by intramembrane domains are graphically depicted by altered membrane curvature.