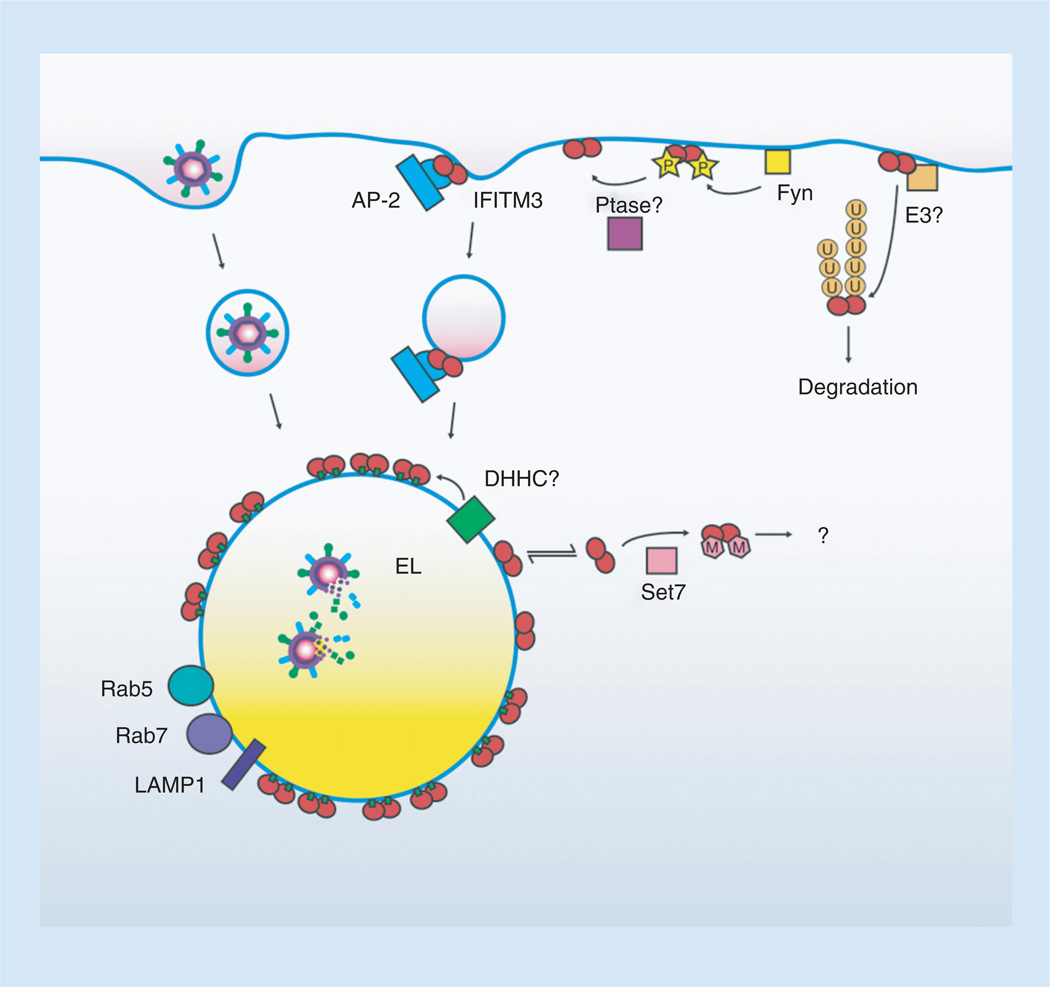
Figure 3. IFITM3 is controlled by multiple post-translational modifications.
IFITM3, depicted as a dimer, is initially localized to the plasma membrane. Tyrosine P (yellow stars) by Fyn retains IFITM3 at the plasma membrane by preventing its interaction with the AP-2 endocytic adaptor complex. Unknown Ptase dynamically remove phosphorylation allowing its endocytosis and targeting to endolysosomes where virus is degraded. Alternatively, unphosphorylated IFITM3 can be targeted for ubiquitination (U, orange circles) by an as yet unidentified E3 ubiquitin ligase resulting in its mislocalization and degradation. Palmitoylation (small green boxes) of IFITM3 by a currently unidentified DHHCanchors IFITM3 to membranes, promotes its clustering, and is necessary for IFITM3 antiviral activity. The location of this palmitoyltransferase activity is currently unknown but is depicted at the endolysosomes. A portion of nonpalmitoylated IFITM3 is cytoplasmic and may be the fraction of IFITM3 that encounters cytoplasmic Set7. IFITM3 methylation (M, pink polygons) by Set7 decreases antiviral activity through an unknown mechanism.
EL: Endolysosome; P: Phosphorylation; Ptase: Phosphatase.