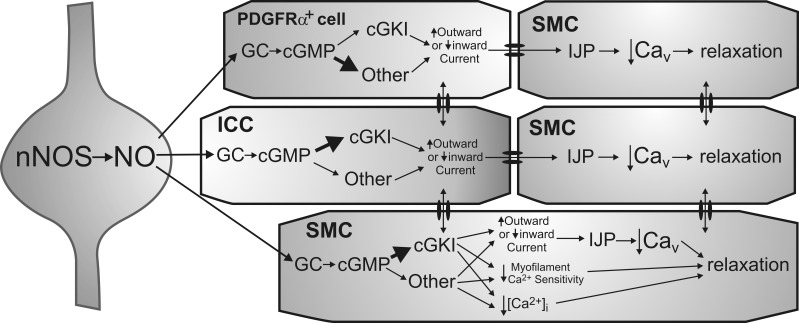
Fig. 10.
Diagram depicting proposed pathways underlying nitrergic neuromuscular transmission in the mouse IAS. Shown is an inhibitory motor neuron that synthesizes and releases nitric oxide (NO) via nNOS (left). Postjunctional effector cells include PDGFRα+ cells (top), ICC (middle), and SMC (bottom and right), which constitute the “SIP” syncytium. Both cGKI-dependent and -independent pathways are depicted. The immediate receptor for NO is GC resulting in cGMP formation. Numerous downstream mediators may then be involved in the actions of cGMP. In ICC, cGKI is suggested to be the initial downstream mediator (thick arrow) since single nitrergic inhibitory junction potentials (IJPs) are absent from the colon of mice lacking cGKI in ICC (29). In contrast, cGKI-independent pathways may predominate in PDGFRα+ cells (thick arrow) since gene expression of cGKI was least in these cells. Both cell types are shown coupled to SMC via gap junctions as previously described (18, 22, 23, 27). There is also evidence that ICC and PDGFRα+ cells may couple to one another (27). Hyperpolarizing currents generated by interstitial cells (via either an increase in outward current or a decrease in inward current) are transmitted to adjacent SMC. This results in IJPs that cause relaxation via the closing of voltage-dependent calcium channels (Cav). SMC are shown at the greatest distance from nerve terminals to depict their role in more slowly developing responses that require multiple stimuli. cGKI is proposed to be the predominant mediator of cGMP effects in SMC (thick arrow) since gene and protein expression of cGKI was greatest in these cells. Unlike ICC and PDGFRα+ cells, relaxation may also be initiated in SMC via mechanisms that do not require a change in membrane potential, e.g., changes in myofilament sensitivity to Ca2+ as well as other mechanisms that reduce intracellular Ca2+ concentration ([Ca2+]i) in a voltage-independent manner. However, since both phasic activity and tone are blocked in the mouse IAS with the Cav blocker nifedipine (6), membrane potential-dependent mechanisms clearly play a pivotal role in the regulation of contraction in this muscle.