Abstract
Vertical sleeve gastrectomy (VSG) is currently one of the most effective treatments for obesity. Despite recent developments, the underlying mechanisms that contribute to the metabolic improvements following bariatric surgery remain unresolved. VSG reduces postprandial intestinal triglyceride (TG) production, but whether the effects of VSG on intestinal metabolism are related to metabolic outcomes has yet to be established. The lipid synthesis enzyme acyl CoA:monoacylglycerol acyltransferase-2 (Mogat2; MGAT2) plays a crucial role in the assimilation of dietary fat in the intestine and in regulation of adiposity stores as well. Given the phenotypic similarities between VSG-operated and MGAT2-deficient animals, we reasoned that this enzyme could also have a key role in mediating the metabolic benefits of VSG. However, VSG reduced body weight and fat mass and improved glucose metabolism similarly in whole body MGAT2-deficient (Mogat2−/−) mice and wild-type littermates. Furthermore, along with an increase in energy expenditure, surgically naive Mogat2−/− mice had altered macronutrient preference, shifting preference away from fat and toward carbohydrates, and increased locomotor activity. Collectively, these data suggest that the beneficial effects of VSG on body weight and glucose metabolism are independent of MGAT2 activity and rather that they are separate from the effects of MGAT2 deficiency. Because MGAT2 inhibitors are proposed as a pharmacotherapeutic option for obesity, our data suggest that, in addition to increasing energy expenditure, shifting macronutrient preference away from fat could be another important mechanism by which these compounds could contribute to weight loss.
Keywords: vertical sleeve gastrectomy, acyl-CoA:monoacylglycerol acyltransferase-2, macronutrient preference, glucose homeostasis, lipid homeostasis
surgical interventions are currently the most effective treatments of obesity (15). Vertical sleeve gastrectomy (VSG), a bariatric procedure that reduces stomach volume by ∼80% without surgical intestinal alteration, induces weight loss and improves glucose tolerance in humans and rodents (8, 17, 18, 24). Although VSG was developed to reduce food intake via restricted stomach capacity, converging data suggest that restriction alone cannot account for either the weight loss or the potent metabolic benefits of VSG (20). Indeed, recent data suggest that important molecular and/or signaling mechanisms must underlie the effects of this procedure (16).
VSG is also associated with reduced postprandial triglyceride levels that are due to reduced intestinal triglyceride (TG) production and not lipid malabsorption (19). Thus, clearly, intracellular lipid handling is being altered by VSG. However, whether the effects of VSG on intestinal lipid metabolism are related to weight loss or improved glucose homeostasis remains unknown. One potential molecular link between intestinal lipid handling and body weight regulation is the triglyceride synthesis enzyme acyl CoA:monoacylglycerol acyltransferase-2 (Mogat2; MGAT2), which is important in the assimilation of dietary fat in the intestine (2, 28). MGAT2 has been hypothesized to be the rate-limiting step of triacylglycerol reassembly in enterocytes (14), and it is responsible for roughly 75% of triacylglycerol synthesis in the intestine, whereas the glycerol 3-phosphate (G3P) pathway accounts for the remaining 25% (5, 6). Importantly, the function of this gene is also related to regulation of both body weight and glucose homeostasis. Loss of whole body or intestinal-specific MGAT2 function protects against diet-induced obesity and glucose intolerance in mice (11, 22, 27). On the one hand, this might be expected, for if triglycerides cannot be resynthesized in the intestine, then their absorption and thus assimilation into adipose stores is limited. However, MGAT2-deficient mice have normal absorption of dietary fat, albeit at a reduced rate of entry of into the circulation (27). Although incompletely understood, the altered kinetics of fat absorption are associated with increased partitioning of dietary fat toward energy dissipation (10, 27).
Like MGAT2 deficiency, VSG-operated rats have normal fat absorption but reduced postprandial triglyceride levels (19). Furthermore, intestinal Mogat2 expression trended to be lower in VSG-treated rats compared with sham-operated controls (19). Finally, both VSG and MGAT deficiency change the secretion of postprandial gut hormones, although changes in glucagon-like peptide-1 (GLP-1) signaling do not appear to be necessary for the metabolic benefit following VSG (12, 24, 27). Given these phenotypic parallels between VSG-operated and MGAT2-deficient mice, we reasoned that VSG decreases intestinal MGAT2 activity, possibly through alterations in nutrient flow or digestion, which contributes to the metabolic effects of VSG surgery. This is supported by the fact that dietary changes alone can have profound effects on intestinal plasticity (4). Therefore, we hypothesized that VSG would have limited or no metabolic benefits in a mouse model of MGAT2 deficiency. To assess this, we performed VSG surgery on a mouse model of whole body MGAT2 deficiency (Mogat2−/− mice) and its wild-type (WT) littermates and measured metabolic and behavioral outcomes. Because VSG alters macronutrient preference (25), we also investigated whether MGAT2 deficiency independently alters macronutrient preference.
MATERIALS AND METHODS
Protocols
The Institutional Animal Care and Use Committee (IACUC) at the University of Cincinnati approved all animal protocols.
Mice
Age-matched experimental male WT and Mogat2−/− littermates, obtained by partnering Mogat2+/− mice, were genotyped as described previously (27). Three-week-old experimental mice were weaned and subsequently housed individually. Mice were maintained at the Metabolic Diseases Institute of the University of Cincinnati on a 12:12-h light-dark cycle at 25 ± 1°C and 50–60% humidity.
Cohort 1.
From weaning, male WT (n = 9) and Mogat2−/− (n = 10) mice had ad libitum access to water and a low-fat chow diet (LM-485 no. 7012: 25% kcal from protein, 58% kcal from carbohydrate, and 17% kcal from fat, plus 3.1 kcal/g metabolizable energy; Harlan-Teklad, Madison, WI) through an in-cage dispenser.
Cohort 2.
From weaning, male WT (n = 16) and Mogat2−/− (n = 16) mice had ad libitum access to water and a 17% low-fat chow diet. From postnatal day 52 onward, mice were provided with a semipurified, lard-based high-fat diet [HFD; D12492: 20% kcal from protein (from casein), 20% kcal from carbohydrate (from corn starch and sucrose), and 60% kcal from fat (from lard), plus 5.24 kcal/g metabolizable energy; Research Diets, New Brunswick, NJ].
Body Weight and Food Intake
After weaning, the body weight of cohort 1 was followed serially, and home cage chow intake was measured during weeks 17 and 18. Feed efficiency during this period was calculated as the change in body weight (g) per 100 kcal ingested. Fat and lean mass were measured in conscious mice using an EchoMRI analyzer (Echo Medical Systems, Houston, TX) at 28 wk of age.
Indirect Calorimetry
For measurements of energy expenditure, respiratory exchange ratio (RER), and physical activity, mice were placed in an indirect calorimetry system for 7 days (TSE Systems, Chesterfield, MO). Only data from the last 4 days were used for data comparisons. Energy expenditure was plotted against body mass at the onset of the metabolic studies and analyzed using regression analysis (21). RER was calculated by dividing carbon dioxide production with oxygen consumption (V̇co2/V̇o2).
Food Preference
Food preference was assessed in cohort 1 using two different paradigms. First, three pure macronutrient diets [TD.02521 (3.3 kcal/g carbohydrate, 0.1% calories protein, 99.9% calories carbohydrate, and 0% calories fat), TD.02522 (6.9 kcal/g fat, 0.1% calories protein, 1.3% calories carbohydrate, and 98.6% calories fat), and TD02523 (3.2 kcal/g protein, 96.1% calories protein, 1.4% calories carbohydrate, and 2.6% calories fat); Harlan-Teklad] were presented in separate containers simultaneously for 9 days (postnatal days 102–111). Nutrient intake was monitored daily, and data are depicted for the final 7 days. Second, to assess a possible preference for caloric density, mice were offered two liquid diets simultaneously, regular Ensure Plus (1.41 kcal/g, 29% from fat; Abbott Nutrition) and Ensure Plus that had been diluted by 50% with water, and intake of both diets was recorded over 48 h (postnatal days 164–166). After 24 h, fresh bottles were provided, and bottle position was switched to prevent a side bias. Mice were preexposed to undiluted Ensure on two separate occasions before the start of the experiment to prevent novelty-induced effects.
Vertical Sleeve Gastrectomy
After 14 wk of HFD consumption, mice from cohort 2 were counterbalanced based on fat and lean mass and assigned to surgical groups (sham operation or VSG). Surgeries were performed as described previously (16, 24), and the same surgeons conducted all surgeries. Briefly, the lateral 80% of the stomach was excised, leaving a tubular gastric remnant in continuity with the esophagus superiorly and the pylorus and duodenum inferiorly. The VSG sham procedure involved analogous isolation of the stomach followed by manual application of pressure with blunt forceps along a vertical line between the esophageal sphincter and the pylorus. The surgical cohorts included sham-operated WT (WT-SHAM), VSG-operated WT (WT-VSG), sham-operated Mogat2−/− (Mogat2−/−-SHAM), and VSG-operated Mogat2−/− (Mogat2−/−-VSG) mice.
Pre- and Postoperative Care
Mice were preexposed to a liquid diet (Osmolite OneCal) before surgery, provided a liquid diet overnight prior to surgery, and then maintained on the liquid diet for the first 3 postoperative days, after which they were transitioned back to the HFD. Subcutaneous injections of Metacam (0.25 mg/100 g body wt once daily for 3 days) and warm saline (1 ml daily for 3 days) were given to all mice following surgery. Complications of surgery resulting in postoperative death or removal of mice from the study, mainly within the first week following surgery, yielded final group numbers of eight WT-SHAM, four WT-VSG, eight Mogat2−/−-SHAM, and four Mogat2−/−-VSG. Mice were removed from the experiment and euthanized based on IACUC criteria, which included excessive body weight loss (>35% of presurgical body weight), poor overall condition, piloerection, and hunched posture. Data from these mice were not included in any of the analyses.
Postmortem analysis of the excluded mice confirmed complications associated with postoperative mortality, including bleeding, infection, and gastric obstruction (16, 24). Experimental mice used for data collection were checked at least once/wk throughout the remainder of the study to ensure overall health.
Postsurgical Body Weight, Food Intake, and Body Composition
Body weight was monitored daily for the first week following surgery and weekly thereafter. Body composition was assessed using an EchoMRI analyzer (Houston, TX) 1 day before and 12 wk following surgery.
Oral Glucose Tolerance Test
Ten weeks following surgery, 5-h-fasted mice were given a 200-μl oral bolus of 25% dextrose at 1300. Blood glucose was assessed at baseline (0 min) and 15, 30, 60, and 120 min via hand-held glucose analyzer (Accuchek; Roche Diagnostics, Indianapolis, IN). Glucose area under the curve (AUC) was calculated using the ΔAUC method.
Oral Fat Tolerance Test
Sixteen weeks following surgery, 5-h-fasted mice were given a 200-μl oral bolus of olive oil at 1300. Plasma samples were obtained from the tail vein before (0) and 60, 120, 240, and 480 min after olive oil gavage for determination of plasma triglyceride levels.
Statistics
All data are presented as means ± SE. Data were analyzed using Statistica 10 (StatSoft, Tulsa, OK). Specifically, data were analyzed by analysis of variance (ANOVA) and ANOVA for repeated measurements where necessary and followed by a Tukey's HSD post hoc test when appropriate to examine significant overall interactions. The null hypothesis was rejected at the 0.05 level.
RESULTS
MGAT2 Deficiency Does Not Alter Energy Balance on a Normal Low-Fat Diet
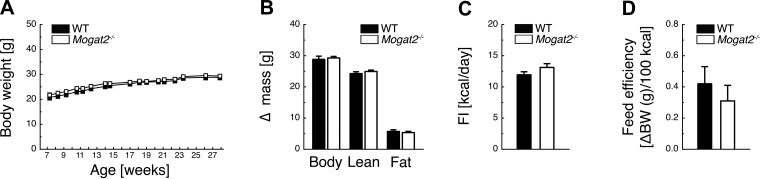
During consumption of a low-fat diet (17% kcal from fat), Mogat2−/− mice had similar body weight growth, accumulation of body, lean, and fat mass, food intake, and feed efficiency as WT controls (Figs. 1, A–D).
Fig. 1.
Acyl-CoA:monoacylglycerol acyltransferase-2 (MGAT2) deficiency does not alter energy balance during low-fat chow feeding. Low-fat diet (17% kcal from fat)-fed Mogat2−/− mice demonstrate similar body weight evolution (A) and similar accumulation of body, lean, and fat mass (B) at 28 wk of age compared with wild-type (WT) controls, with no significant differences in food intake (C) or feed efficiency (D) during weeks 17 and 18; n = 9 WT and 10 Mogat2−/−. BW, body weight.
MGAT2 Deficiency Alters Macronutrient Preference
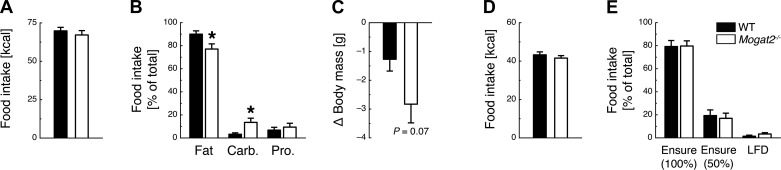
During the 7-day macronutrient selection assessment paradigm, total caloric intake was similar between Mogat2−/− and WT mice [t(1, 17) = 0.72, P = 0.48; Fig. 2A]. Mogat2−/− mice had lower preference for fat [t(1, 17) = 2.36, P < 0.05], increased preference for carbohydrates [t(1, 17) = 2.56, P < 0.05], and no difference in preference for protein [t(1, 17) = 0.61, P = 0.55] compared with WT mice (Fig. 2B). During the macronutrient selection paradigm, body weight loss trended to be greater in Mogat2−/− mice than WT mice [t(1, 17) = 1.70, P = 0.07; Fig. 2C].
Fig. 2.
MGAT2 deficiency alters macronutrient preference but not caloric density preference. A: WT and Mogat2−/− mice consume a similar amount of calories during a 9-day macronutrient preference paradigm consisting of free choice between fat, carbohydrates (Carb.), and protein (Pro.). B and C: Mogat2−/− mice have decreased preference for fat and increased preference for carbohydrates (B) and show a trend toward losing more weight than WT mice during the macronutrient paradigm (C). D and E: WT and Mogat2−/− mice consume a similar amount of total calories during a 2-day caloric density preference paradigm (D) and have no altered preference for undiluted Ensure liquid diet, 50%-diluted Ensure liquid diet, or a low-fat diet (LFD; E). *P < 0.05, WT vs. Mogat2−/−; n = 9 WT and 10 Mogat2−/−.
MGAT2 Deficiency Does Not Alter Preference for Caloric Density
During the 2-day caloric density preference paradigm, total caloric intake was similar between WT and Mogat2−/− [t(1, 17) = 0.85, P = 0.41; Fig. 2D]. Both WT and Mogat2−/− mice preferred undiluted over 50% diluted Ensure and low-fat diet, with no significant differences between the genotypes (P > 0.05; Fig. 2E).
MGAT2 Deficiency Protects Against HFD-Induced Obesity
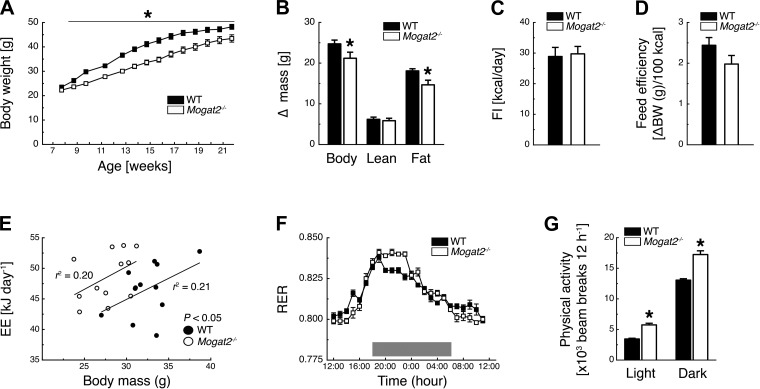
Similar to previous reports, when consuming a HFD (60% kcal from fat) Mogat2−/− mice had less body weight gain than WT controls [time × genotype interaction, F(13, 416) = 6.39, P < 0.05; Fig. 3A]. During this period, Mogat2−/− mice accumulated less total body mass [t(1, 32) = 1.98, P < 0.05] and less fat mass [t(1, 32) = 2.60, P < 0.05] compared with WT controls (Fig. 3B). This difference in body mass accumulation was not explained by differences in caloric intake, as Mogat2−/− mice had similar food intake as WT mice during weeks 13 and 14 [t(1,21) = 0.23, P = 0.82; Fig. 3C]. There was also a trend for feed efficiency to be lower in Mogat2−/− mice [t(1, 32) = 1.72, P = 0.09; Fig. 3D]. As demonstrated previously (10, 27), when energy expenditure was plotted against body weight of each mouse, the regression line of Mogat2−/− mice was significantly elevated over those of WT controls (effect of genotype, P < 0.05; Fig. 3E), indicating higher energy expenditure in Mogat2−/− mice than WT controls. Because the mice were fed a 60% HFD, both WT and Mogat2−/− mice demonstrated RER values of ∼0.8 (Fig. 3F). Interestingly, RER values over 24 h revealed a time × genotype interaction [F(23, 138) = 1.77, P < 0.05] and, similar to a previous study (10), revealed that Mogat2−/− mice oxidized significantly higher proportions of fat than WT mice during the inactive light phase (Fig. 3F). Moreover, Mogat2−/− mice actually had higher RER values than WT mice predominantly during the first half of the dark phase, when mice consume most of their calories (Fig. 3F). Finally, Mogat2−/− mice demonstrated increased locomotor activity during both the light and dark phases [time × genotype interaction, F(1, 12) = 6.92, P < 0.05; Fig. 3G].
Fig. 3.
MGAT2 deficiency protects against high-fat diet (HFD)-induced obesity. A–D: HFD (60% kcal from fat)-fed Mogat2−/− mice demonstrate delayed body weight growth (A) and decreased accumulation of body and fat mass (B) between weeks 8 and 22 compared with WT controls despite no significant differences in food intake (FI; C) or feed efficiency (D) (weeks 13 and 14). E: regression analyses of energy expenditure (EE) vs. body mass of mice. EE per mouse was plotted against the body mass of each mouse at the start of metabolic phenotyping. F and G: Mogat2−/− mice demonstrate lower and higher respiratory exhcnage ratio (RER) during the light phase and first half of the dark phase, respectively (F) and increased physical activity during the light and dark phase compared with WT controls (G). Ten-week-old male mice were acclimated individually to metabolic cages, and experiments were performed for 7 days. EE, RER, and physical activity data were averaged for the last 4 days. Gray area marks dark phase (1800–0600). *P < 0.05, WT vs. Mogat2−/−; n = 16 WT (A–D) and 16 Mogat2−/− (E–G); n = 12 WT and 12 Mogat2−/−.
Effects of VSG on Weight Loss are Independent of MGAT2
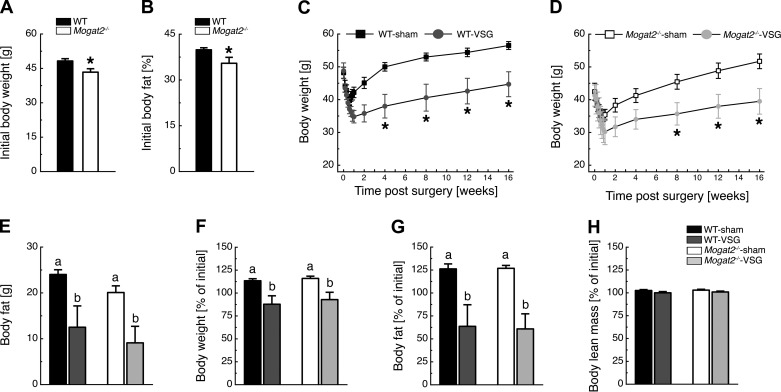
At the time of VSG surgery, after 14 wk of consuming the 60% HFD, Mogat2−/− mice weighed less [t(1, 32) = 2.57, P < 0.05] and had less fat mass [t(1, 32) = 2.58, P < 0.05] than WT mice (Figs. 4, A and B). VSG surgery caused initial weight loss in both WT-VSG and WT-sham mice, but only WT-VSG mice maintained weight loss over time [time × treatment interaction, F(12, 120) = 11.56, P < 0.05; Fig. 4C]. VSG also caused initial weight loss in both Mogat2−/−-VSG and Mogat2−/−-sham mice, with Mogat2−/−-VSG mice maintaining weight loss over time [time × treatment interaction, F(12, 120) = 12.94, P < 0.05; Fig. 4D]. Twelve weeks after VSG surgery, both WT-VSG and Mogat2−/−-VSG mice had ∼11 g less body fat than their sham-operated controls [effect of treatment, F(1, 19) = 26.76, P < 0.05; Fig. 4E]. These observations reflected an ∼25% decrease in body weight [effect of treatment, F(1, 19) = 6.72, P < 0.05; Fig. 4F] and a 60% decrease in fat mass [effect of treatment, F(1, 19) = 34.18, P < 0.05; Fig. 4G] in both WT-VSG and Mogat2−/−-VSG mice compared with sedentary controls. Because both WT-sham and Mogat2−/−-sham mice gained body weight and fat mass, it is important to note that mice, in contrast to humans, continue to add both fat and lean mass throughout their lifespan, and clearly bariatric surgery did not stunt this growth curve. Lean mass was unaffected by genotype or VSG surgery (Fig. 4H).
Fig. 4.
MGAT2 does not contribute to the maintenance of weight loss following vertical sleeve gastrectomy (VSG). HFD (60% kcal from fat)-fed WT and Mogat2−/− mice weighed >40 g (A) and carried >30% of their weight as fat before the surgery (B). Both WT-VSG (C) and Mogat2−/−-VSG mice (D) lost weight and maintained this weight loss relative to sham-operated controls. E–G: 12 weeks after surgery, both WT-VSG and Mogat2−/−-VSG mice had ∼11 g less body fat (E), 25% less body weight (F), and 65% less body fat (G) compared with sham-operated controls, whereas both WT-sham and Mogat2−/−-sham mice gained ∼12% and 25% of presurgical BW and fat, respectively. H: lean mass was unaffected by VSG surgery. *P < 0.05, WT vs. Mogat2−/−, WT-sham vs. WT-VSG, and Mogat2−/−-sham vs. Mogat2−/−-VSG. Different letters indicate significant difference as follows: a,bP < 0.05, effect of surgery; n = 8 WT-sham, 4 WT-VSG, 8 Mogat2−/−-sham, and 4 Mogat2−/−-VSG.
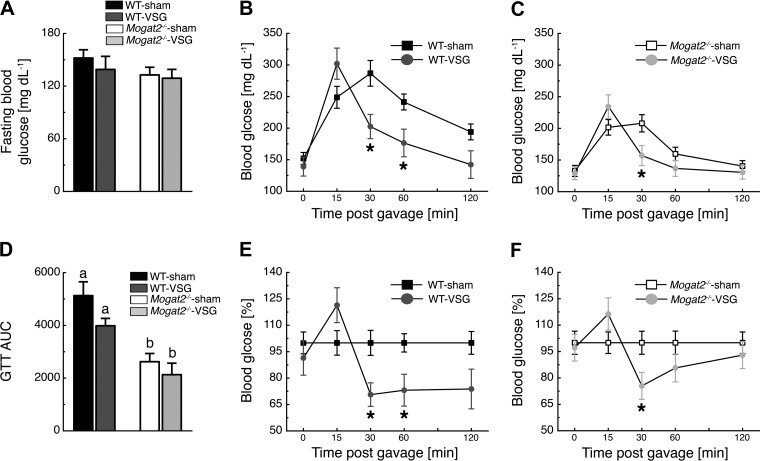
Effects of VSG on Improved Glucose Tolerance are Independent of MGAT2
Ten weeks following VSG surgery, fasting blood glucose levels were similar among the groups (Fig. 5A). After an oral glucose bolus gavage, plasma glucose values in WT mice revealed a time × treatment interaction [F(4, 40) = 10.86, P < 0.05], with WT-VSG mice having lower plasma glucose values than WT-sham mice at 30 and 60 min following the gavage (Fig. 5B). Similar to WT mice, plasma glucose values in Mogat2−/− mice also revealed a time × treatment interaction [F(4, 40) = 14.33; P < 0.05], with Mogat2−/−-VSG mice having lower plasma glucose values than Mogat2−/−-sham mice at 30 min following the gavage (Fig. 5C). Due to the elevated gastric emptying rate after VSG (3), VSG-operated mice had elevated plasma glucose 15 min following the oral glucose load, and AUC values were not statistically different between VSG- and sham-operated mice (Fig. 5D). Of note is that both WT-VSG and Mogat2−/−-VSG mice had ∼20% lower AUC levels compared with sedentary controls (Fig. 5D). AUC values were lower in Mogat2−/− mice than in WT mice independent of treatment [F(1, 20) = 21.11, P < 0.05; Fig. 5D]. Finally, blood glucose values graphed relative to sham-operated controls revealed that WT-VSG and Mogat2−/−-VSG mice showed similar improvements in blood glucose levels (51 ± 16 and 41 ± 2%, respectively, P = 0.58) between t = 15 and t = 30 (Fig. 5, E and F). However, contrary to WT mice, blood glucose differences between Mogat2−/−-sham and Mogat2−/−-VSG disappeared during the last 90 min of the oral glucose tolerance test.
Fig. 5.
MGAT2 does not contribute to the improvements in glucose tolerance following VSG. A: 10 wk following VSG surgery, all experimental groups had similar fasting blood glucose. B and C: both WT-VSG (B) and Mogat2−/−-VSG (C) mice had improved glucose excursions following an oral flat dose of glucose compared with sham-operated controls. D: Mogat2−/− mice had lower glucose area under the curve (AUC) values than WT mice independent of treatment. E and F: blood glucose levels of WT-VSG (E) and Mogat2−/−-VSG (F) mice graphed relative to sham controls. *P < 0.05, WT-sham vs. WT-VSG, Mogat2−/−-sham vs. Mogat2−/−-VSG. Different letters indicate significant difference as follows a,bP < 0.05, effect of genotype; n = 8 WT-sham, 4 WT-VSG, 8 Mogat2−/−-sham, and 4 Mogat2−/−-VSG. GTT, glucose tolerance test.
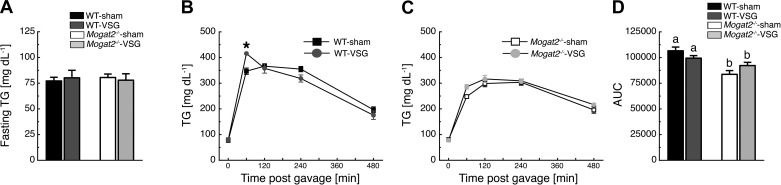
MGAT2 Deficiency Improves Fat Tolerance Independent of VSG
Sixteen weeks following VSG surgery, fasting blood TG levels were similar among the groups (Fig. 6A). After oral administration of an olive oil bolus, plasma TG levels in WT mice revealed a time × treatment interaction [F(4, 36) = 6.15, P < 0.05], with WT-VSG mice having higher plasma TG levels than WT-sham mice at 15 min following the gavage (Fig. 6B). Plasma TG levels did not differ significantly between Mogat2−/−-VSG and Mogat2−/−-sham mice (Fig. 5C). Finally, oral fat tolerance test AUC values were lower in Mogat2−/− mice compared with those of WT mice independent of treatment [F(1, 19) = 12.84, P < 0.05; Fig. 6D].
Fig. 6.
MGAT2 deficiency increases oral fat tolerance independent of VSG. A: 16 wk following VSG surgery, all experimental groups had similar fasting plasma triglycerides (TG). B: following an oral flat dose of olive oil, WT-VSG mice had higher plasma TG levels compared with WT-sham controls at 15 min following the gavage. C: Mogat2−/−-VSG mice had similar TG excursions compared with Mogat2−/−-sham controls. D: Mogat2−/− mice had lower TG AUC values than WT mice independent of treatment. *P < 0.05, WT-sham vs. WT-VSG. Different letters indicate significant difference as follows: a,bP < 0.05, effect of genotype; n = 8 WT-sham, 4 WT-VSG, 8 Mogat2−/−-sham, and 4 Mogat2−/−-VSG.
DISCUSSION
Here, we demonstrate that, similar to our previous work (16, 24), VSG causes significant weight loss and improves glucose tolerance in mice. Based on the metabolic parallels between VSG and MGAT deficiency and trends for VSG-induced alterations in intestinal Mogat2 expression, we hypothesized that loss of MGAT function would significantly blunt the full metabolic effects of VSG on body weight and glucose tolerance. Instead, Mogat2−/− mice responded normally to VSG, indicating that alterations in MGAT2 activity are not necessary to manifest the beneficial effects of VSG surgery.
Humans and rodents display remarkably similar qualitative metabolic improvements following bariatric surgery, including rapid body weight-independent improvements in glucose tolerance, increased insulin secretion, and increased circulatory GLP-1 levels (20). Although there are differences in pattern of body weight and food intake changes postsurgery, it is important to note that both humans and rodents actively defend and maintain a lower body weight following bariatric surgery. Thus, rodents afford us a high-throughput option with dietary control that allows us to explore weight loss-dependent and -independent mechanisms for bariatric surgery.
We replicated and expanded on key elements of the obesity-resistant phenotype observed in Mogat2−/− mice (22, 27). First, we observed normal body weight and fat mass accumulation, food intake, and feed efficiency in low-fat chow-fed WT vs. Mogat2−/− mice. Second, we found that HFD-fed Mogat2−/− mice are protected from HFD-induced obesity due to increases in energy expenditure, as reported previously (10, 27). Importantly, we also report several novel MGAT2 deficiency phenotypes. First, we observed an increase in daily physical activity, which contrasts with previous findings (27). Although the mechanisms underlying this increase in home cage locomotion are unclear, it should be noted that below thermoneutrality, other metabolic processes involved with thermoregulation likely mask the contribution of physical activity to energy expenditure (23). Furthermore, in agreement with a previous study (10), RER was slightly lower in HFD-fed Mogat2−/− mice during the light phase. However, we also observed a slightly higher RER in HFD-fed Mogat2−/− mice during the first half of the dark phase. This suggests that Mogat2−/− mice efficiently switch from utilizing body fat stores to utilizing dietary carbohydrates during the early active phase, when feeding predominantly occurs.
We also observed that MGAT2 deficiency alters macronutrient preference. Specifically, Mogat2−/− mice have decreased fat and increased carbohydrate preference, suggesting that when mice are given a choice, MGAT2 deficiency shifts preference away from fat and toward carbohydrates, potentially as an attempt to minimize unabsorbed fat. Importantly, this was not simply an effort to avoid calorically dense foods, as WT and Mogat2−/− mice equally preferred undiluted to diluted Ensure, a mixed-macronutrient liquid diet. Although VSG surgery does not appear to alter intestinal lipid storage (19), it has been demonstrated that MGAT2 deficiency shifts the distribution of enterocyte lipid clusters toward the distal part of the intestine (27). Collectively, these data indicate an important link between intestinal lipid processing and/or signaling and central nervous system regulation of feeding. MGAT2 inhibitors have been proposed as a pharmacotherapeutic option for obesity, and the present data highlight that along with increasing energy expenditure, shifting macronutrient preference could be another important mechanism by which these drugs could contribute to weight loss.
Recently, we demonstrated that increased circulating total bile acids (9, 13, 16) and signaling through the farnesoid X receptor (Fxr) are required for the metabolic improvements produced by VSG (16). In addition to their ability to signal via nuclear and cell surface receptors, bile acids are critical for the normal absorption of lipids from the gastrointestinal tract. Interestingly, similar to Mogat2−/− mice, Fxr−/− mice have a decreased preference for fat compared with WT littermates (16). It remains unknown whether the improved intestinal-specific lipid handling after VSG is prevented by FXR deficiency, but it is clear that VSG induces important intestinal signaling events crucial to the success of the surgery. Future research will clarify the key molecular mechanism response for the changes in lipid handling after VSG.
The present data also confirm our prior reports indicating that VSG results in sustained body weight loss, has little to no effect on lean tissue mass, decreases fat mass, and improves glucose tolerance (16, 24). Importantly, all of these effects of VSG surgery appear not to require MGAT2. Interestingly, blood glucose differences between Mogat2−/−-sham and Mogat2−/−-VSG did disappear during the last 90 min of the oral glucose tolerance test. However, this observation likely resulted from basal improvements in glucose metabolism during MGAT2 deficiency rather than from differential responses to VSG. Of note, despite the complete absence of MGAT2, Mogat2−/− mice retain ∼50% of MGAT function (27), suggesting the existence of an as-yet unidentified MGAT protein or increased efficiency through the G3P pathway. In addition, it has been demonstrated that acyl CoA:diacylglycerol acyltransferase-1 also has MGAT activity (29). Thus, although MGAT2 is not necessary for VSG surgery to exert its metabolic effects, we cannot exclude the possibility that general MGAT function or G3P plays a role.
Total or intestine-specific MGAT2 deficiency increases plasma levels of GLP-1 (11, 27), a peptide hormone released from L cells of the small intestine in response to nutrient ingestion. GLP-1 stimulates insulin secretion, decreases food intake, and improves various aspects of glucose tolerance in addition to the augmentation of insulin secretion (1). It has been hypothesized that GLP-1 action underlies the metabolic improvements induced by bariatric surgeries. Although we did not measure plasma GLP-1 in this study, we and others have demonstrated recently that both VSG (24) and Roux-en-Y gastric bypass (7, 26) surgery are effective in mouse models of GLP-1 receptor deficiency. Thus, our observation that VSG has similar efficacy in WT and Mogat2−/− mice, which have elevated plasma GLP-1 levels, strengthens the conclusion that GLP-1 action is not required for VSG surgery to exert its potent effects on several metabolic and behavioral outcomes.
In summary, we find independent effects of MGAT2 deficiency and vertical sleeve gastrectomy on metabolic outcomes in the mouse. However, in our efforts to understand the role of MGAT2, we did find that MGAT2 regulates macronutrient preference. These data suggest an additional novel mechanism by which the proposed MGAT2 inhibitors work to treat obesity and its associated comorbidities.
GRANTS
This work was supported by research grants from Ethicon Surgical Care and the National Institute of Diabetes and Digestive and Kidney Diseases (DK-017844 to S. C. Woods; DK-056084 to R. V. Farese, Jr.; DK-54080, DK-82480, DK-54890, and DK-056863 to R. J. Seeley; and DK-082480 to D. A. Sandoval). J. D. Mul was supported by an American Society for Metabolic and Bariatric Surgery grant. D. P. Begg was supported by a National Health and Medical Research Council of Australia Early Career Fellowship (1013264). D. A. Sandoval is the corresponding author and guarantor.
DISCLOSURES
The authors declare the following competing financial interests: D. A. Sandoval receives research support from Novo Nordisk and Boehringer-Ingelheim. R. J. Seeley is a paid speaker for Novo Nordisk and Merck. R. J. Seeley serves on scientific advisory boards for Novo Nordisk, Novartis, Angiochem, Zealand, Takeda, Eli Lilly, Boehringer-Ingelheim, Eisai, and Forest Pharmaceuticals and receives research support from Novo Nordisk, Ethicon Endo-Surgery, Ablaris, Boehringer-Ingelheim, and Zealand. Also, Dr. Seeley has equity in Zafgen.
AUTHOR CONTRIBUTIONS
J.D.M., D.P.B., and D.A.S. conception and design of research; J.D.M., D.P.B., A.M.H., J.W.P., and J.S. performed experiments; J.D.M., D.P.B., A.M.H., J.W.P., and J.S. analyzed data; J.D.M., D.P.B., S.C.W., R.V.F.J., R.J.S., and D.A.S. interpreted results of experiments; J.D.M. and D.P.B. prepared figures; J.D.M. and D.P.B. drafted manuscript; J.D.M., D.P.B., S.C.W., R.V.F.J., R.J.S., and D.A.S. edited and revised manuscript; J.D.M., D.P.B., A.M.H., J.W.P., J.S., S.C.W., R.V.F.J., R.J.S., and D.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kathleen Smith, Kenneth Parks, Mouhamadoul Toure, Jose Berger, and Alfor Lewis for their surgical expertise in conducting the VSG and sham surgeries, Brad Chambers for conducting the metabolic phenotyping studies, and all members of the Seeley and Sandoval laboratories for helpful discussion and comments.
Present address of J. D. Mul: Joslin Diabetes Center, Harvard Medical School, Boston, MA 02215.
Present address of R. V. Farese, Jr.: Harvard School of Public Health, Boston, MA 02215.
REFERENCES
- 1.Barrera JG, Sandoval DA, D'Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol 7: 507–516, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao J, Hawkins E, Brozinick J, Liu X, Zhang H, Burn P, Shi Y. A predominant role of acyl-CoA:monoacylglycerol acyltransferase-2 in dietary fat absorption implicated by tissue distribution, subcellular localization, and up-regulation by high fat diet. J Biol Chem 279: 18878–18886, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Chambers AP, Smith EP, Begg DP, Grayson BE, Sisley S, Greer T, Sorrell J, Lemmen L, LaSance K, Woods SC, Seeley RJ, D'Alessio DA, Sandoval DA. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab 306: E424–E432, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dailey MJ. Nutrient-induced intestinal adaption and its effect in obesity. Physiol Behav. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kayden HJ, Senior JR, Mattson FH. The monoglyceride pathway of fat absorption in man. J Clin Invest 46: 1695–1703, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattson FH, Volpenhein RA. The Digestion and Absorption of Triglycerides. J Biol Chem 239: 2772–2777, 1964. [PubMed] [Google Scholar]
- 7.Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab 3: 191–201, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mul JD, Begg DP, Alsters SI, Haaften G, Duran KJ, D'Alessio DA, le Roux CW, Woods SC, Sandoval DA, Blakemore AI, Cuppen E, van Haelst MM, Seeley RJ. Effect of vertical sleeve gastrectomy in melanocortin receptor 4-deficient rats. Am J Physiol Endocrinol Metab 303: E103–E110, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD, Dexheimer PJ, Aronow B, Seeley RJ, Kohli R. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring) 22: 390–400, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson DW, Gao Y, Spencer NM, Banh T, Yen CL. Deficiency of MGAT2 increases energy expenditure without high-fat feeding and protects genetically obese mice from excessive weight gain. J Lipid Res 52: 1723–1732, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson DW, Gao Y, Yen MI, Yen CL. Intestine-specific deletion of Acyl-CoA:monoacylglycerol acyltransferase (MGAT) 2 protects mice from diet-induced obesity and glucose intolerance. J Biol Chem 289: 17338–17349, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okawa M, Fujii K, Ohbuchi K, Okumoto M, Aragane K, Sato H, Tamai Y, Seo T, Itoh Y, Yoshimoto R. Role of MGAT2 and DGAT1 in the release of gut peptides after triglyceride ingestion. Biochem Biophys Res Commun 390: 377–381, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, Auwerx J, Goldfine AB. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 17: 1671–1677, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phan CT, Tso P. Intestinal lipid absorption and transport. Front Biosci 6: D299–D319, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab 93: S89–S96, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Perez HE, Sandoval DA, Kohli R, Backhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509: 183–188, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 366: 1567–1576, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stefater MA, Perez-Tilve D, Chambers AP, Wilson-Perez HE, Sandoval DA, Berger J, Toure M, Tschop M, Woods SC, Seeley RJ. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology 138: 2426–2436, 2436.e1–2436.e13, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stefater MA, Sandoval DA, Chambers AP, Wilson-Perez HE, Hofmann SM, Jandacek R, Tso P, Woods SC, Seeley RJ. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology 141: 939–949.e1–939-949.e4, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stefater MA, Wilson-Perez HE, Chambers AP, Sandoval DA, Seeley RJ. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev 33: 595–622, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tschöp MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, Eckel RH, Farese RV, Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Müller TD, Munzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nat Methods 9: 57–63, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuchida T, Fukuda S, Aoyama H, Taniuchi N, Ishihara T, Ohashi N, Sato H, Wakimoto K, Shiotani M, Oku A. MGAT2 deficiency ameliorates high-fat diet-induced obesity and insulin resistance by inhibiting intestinal fat absorption in mice. Lipids Health Dis 11: 75, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virtue S, Even P, Vidal-Puig A. Below thermoneutrality, changes in activity do not drive changes in total daily energy expenditure between groups of mice. Cell Metab 16: 665–671, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson-Pérez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, Drucker DJ, Pérez-Tilve D, Seeley RJ. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes 62: 2380–2385, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson-Pérez HE, Chambers AP, Sandoval DA, Stefater MA, Woods SC, Benoit SC, Seeley RJ. The effect of vertical sleeve gastrectomy on food choice in rats. Int J Obes (Lond) 37: 288–295, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye J, Hao Z, Mumphrey MB, Townsend RL, Patterson LM, Stylopoulos N, Münzberg H, Morrison CD, Drucker DJ, Berthoud HR. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol 306: R352–R362, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yen CL, Cheong ML, Grueter C, Zhou P, Moriwaki J, Wong JS, Hubbard B, Marmor S, Farese RV., Jr Deficiency of the intestinal enzyme acyl CoA:monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nat Med 15: 442–446, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yen CL, Farese RV., Jr MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J Biol Chem 278: 18532–18537, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Yen CL, Monetti M, Burri BJ, Farese RV., Jr The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J Lipid Res 46: 1502–1511, 2005. [DOI] [PubMed] [Google Scholar]