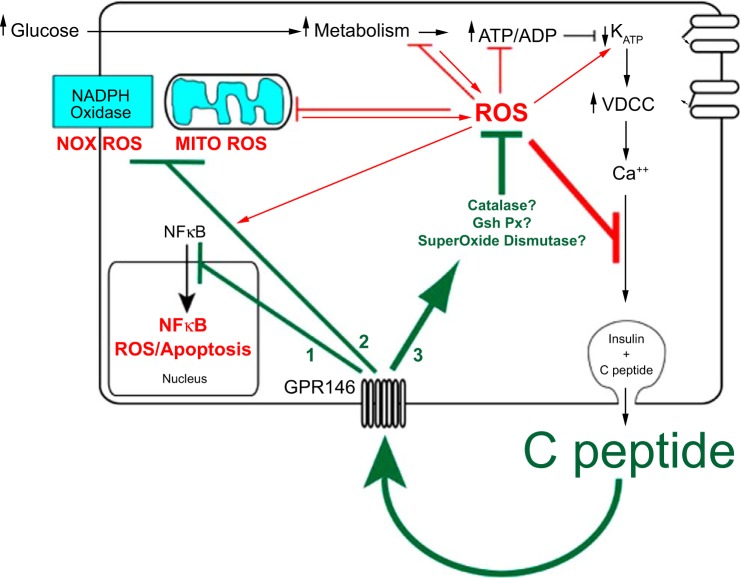
Fig. 3.
Autocrine C-peptide model underlying β-cell adaptation to oxidative stress. The centerpiece of functional β-cell mass is insulin secretion, which is cosecreted in equimolar amounts with C-peptide. Autocrine action of C-peptide, which protects functional β-cell mass, should therefore provide a buffer of protection for its own secretion as well as that of insulin. In addition to binding to its receptor GPR146 for such autocrine actions, C-peptide is predicted to regulate at least 3 distinct pathways in the β-cell: 1) deactivation of the NF-κB pathway, which protects against apoptosis; 2) inhibition of pathways that generate ROS including the plasmalemma NADPH oxidase and the mitochondrial electron transport chain; 3) activation of pathways that catalyze the degradation of ROS by activating antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase. In the absence of these autocrine actions of C-peptide, more prolonged and higher accumulation of ROS and apoptosis would accelerate loss of β-cell functional mass. This suggests a tipping point in loss of functional β-cell mass where the loss in C-peptide secretion and its autocrine protection results in a downward spiral of both secretion and protection, ultimately leading to few if any β-cells.