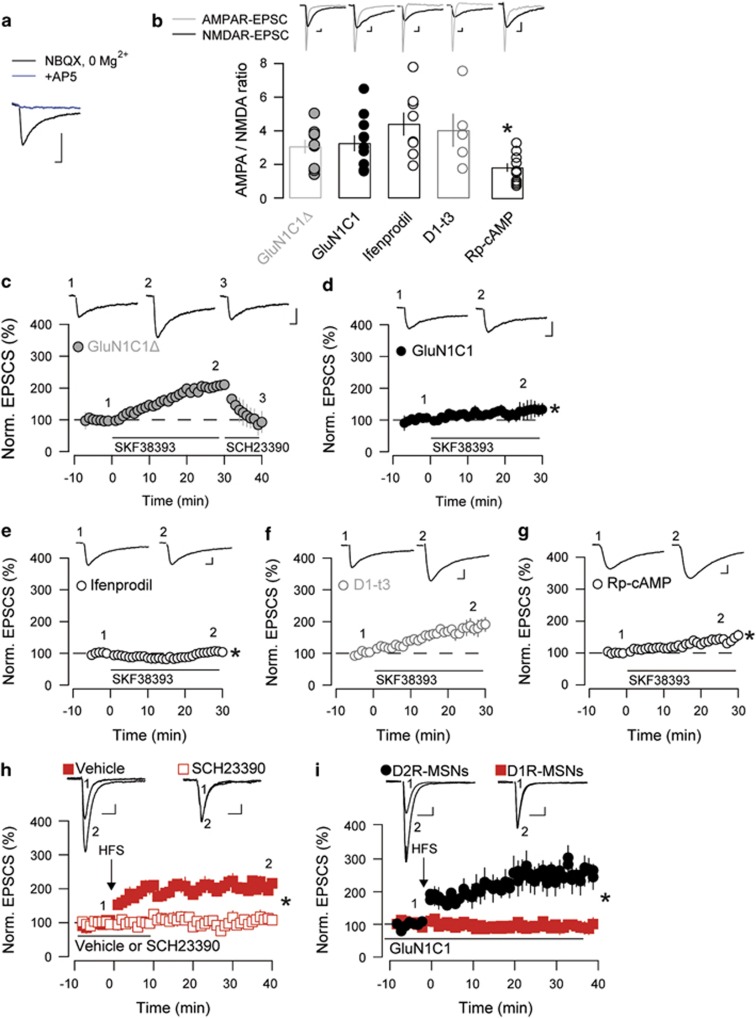
Figure 4.
Dopamine D1 receptor (D1R)/GluN1 complexes control long-term synaptic plasticity in D1R-medium spiny neurons (MSNs). (a) NMDAR-mediated excitatory post synaptic currents (EPSCs) were pharmacologically isolated (blockade of AMPAR or NMDAR-EPSC by NBQX and by AP5, respectively). (b) Example traces and quantification of AMPA/NMDAR ratio after AMPAR- and NMDAR-EPSCs were recorded successively (before and after pharmacological isolation of the NMDAR-EPSC) in presence of GluN1C1, or GluN1C1Δ, D1-t3, RpcAMP in the patch pipette or bath application of the GluN2B-containing NMDAR antagonist, ifendprodil (n=6–10). Students unpaired t-test, *P<0.05 different from control (GluN1C1Δ). (c) The potentiation of NMDAR-mediated EPSCs by SKF38393 observed in the presence of GluN1C1Δ in the internal solution is reversed by bath application of a D1R antagonist (SCH-23390 10 μM; n=9). (d) In the presence of GluN1C1, SKF38393 no longer potentiates NMDAR-EPSCs (n=10). (e) Ifenprodil prevents the SKF38393-induced potentiation of NMDAR-mediated EPSCs (n=8), whereas the D1-t3 peptide (n=5) has no effect (f). (g) RpcAMP partially, but significantly, alters the potentiation NMDAR-mediated EPSCs by a D1R agonist (n=12). (h) The D1R antagonist (SCH-23390 10 μM; n=6) prevents high-frequency stimulation-induced (HFS, 100 Hz) long-term potentiation (LTP) of AMPAR-EPSCs recorded in D1R-MSNs (n=6). (i) GluN1C1 prevents HFS-LTP in D1R-MSNs (n=12) but not in D2R-MSNs (n=6). (a–i) MSNs were identified using drd1a-eGFP, drd1a-tdTomato or drd2-eGFP transgenic mice. *P<0.05; Students unpaired t-test when percent variation from baseline during the last 5 min is different from control condition. Scale bars, 10 ms, 20 pA.