Abstract
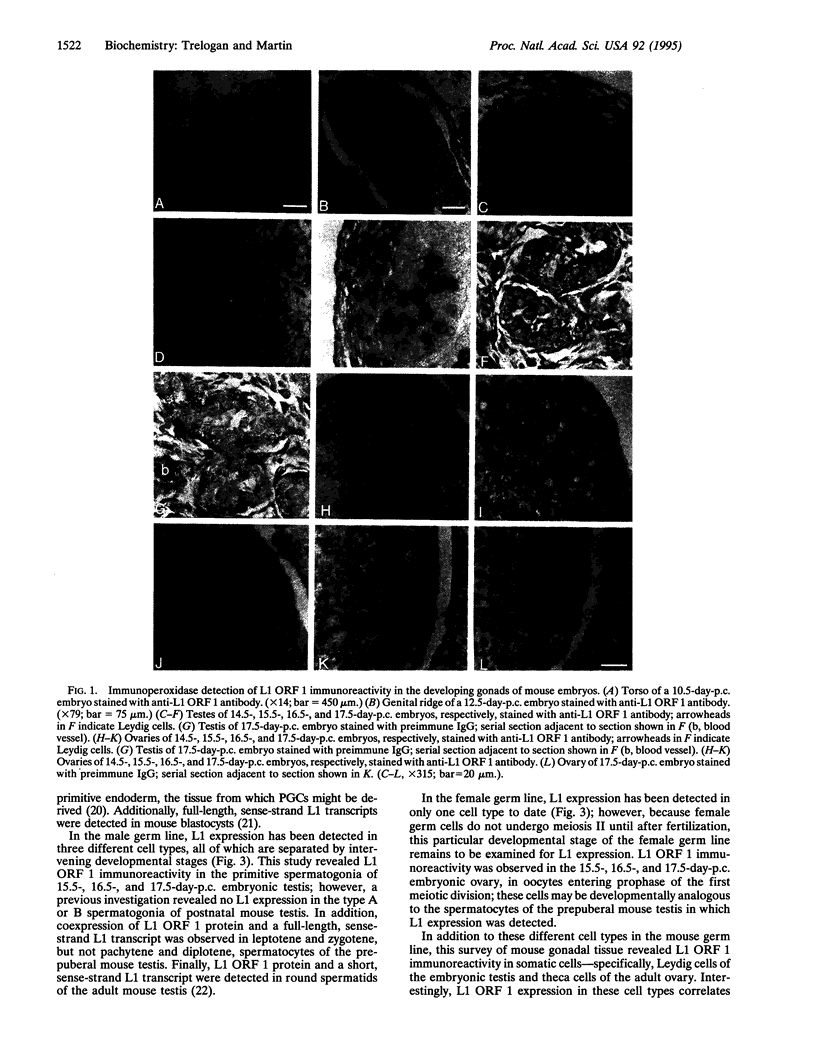
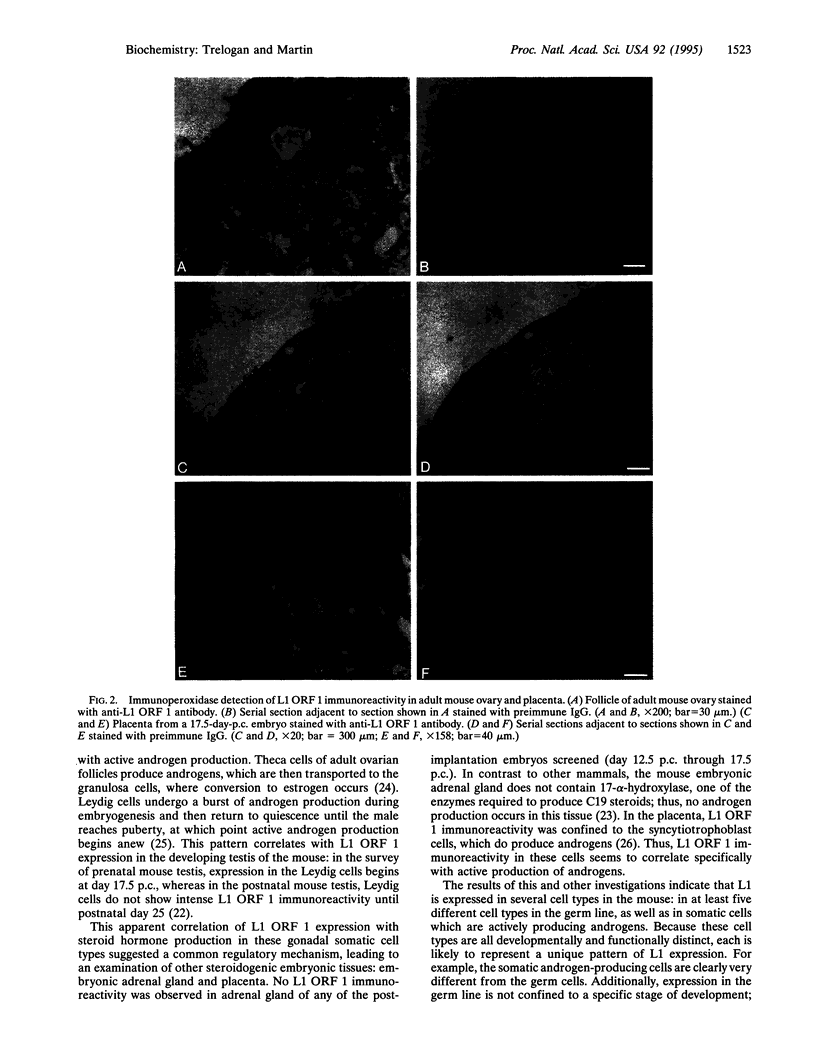
LINE-1 (L1) has achieved its status as a middle repetitive DNA family in mammalian genomes by duplicative transposition. Although transposition may occur in any cell type, expression and transposition of a full-length functional element in the germ line are necessary for evolutionarily significant propagation of L1. An immunohistochemical analysis of adult mouse ovaries and mouse postimplantation embryos revealed expression of L1 open reading frame 1 in the germ line as well as in steroidogenic tissues. These results demonstrate that L1 expression is controlled by a tightly regulated temporal and spatial program of events during development and imply that multiple loci of L1 in the mouse genome are active for expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branciforte D., Martin S. L. Developmental and cell type specificity of LINE-1 expression in mouse testis: implications for transposition. Mol Cell Biol. 1994 Apr;14(4):2584–2592. doi: 10.1128/mcb.14.4.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIQUOINE A. D. The identification, origin, and migration of the primordial germ cells in the mouse embryo. Anat Rec. 1954 Feb;118(2):135–146. doi: 10.1002/ar.1091180202. [DOI] [PubMed] [Google Scholar]
- Deragon J. M., Sinnett D., Labuda D. Reverse transcriptase activity from human embryonal carcinoma cells NTera2D1. EMBO J. 1990 Oct;9(10):3363–3368. doi: 10.1002/j.1460-2075.1990.tb07537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg M., Snow M. H., McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development. 1990 Oct;110(2):521–528. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- Gomperts M., Garcia-Castro M., Wylie C., Heasman J. Interactions between primordial germ cells play a role in their migration in mouse embryos. Development. 1994 Jan;120(1):135–141. doi: 10.1242/dev.120.1.135. [DOI] [PubMed] [Google Scholar]
- Holmes S. E., Dombroski B. A., Krebs C. M., Boehm C. D., Kazazian H. H., Jr A new retrotransposable human L1 element from the LRE2 locus on chromosome 1q produces a chimaeric insertion. Nat Genet. 1994 Jun;7(2):143–148. doi: 10.1038/ng0694-143. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I., Pelliniemi L. J. Fetal Leydig cells: cellular origin, morphology, life span, and special functional features. Proc Soc Exp Biol Med. 1992 Nov;201(2):125–140. doi: 10.3181/00379727-201-43493. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Wong C., Youssoufian H., Scott A. F., Phillips D. G., Antonarakis S. E. Haemophilia A resulting from de novo insertion of L1 sequences represents a novel mechanism for mutation in man. Nature. 1988 Mar 10;332(6160):164–166. doi: 10.1038/332164a0. [DOI] [PubMed] [Google Scholar]
- Kingsmore S. F., Giros B., Suh D., Bieniarz M., Caron M. G., Seldin M. F. Glycine receptor beta-subunit gene mutation in spastic mouse associated with LINE-1 element insertion. Nat Genet. 1994 Jun;7(2):136–141. doi: 10.1038/ng0694-136. [DOI] [PubMed] [Google Scholar]
- Kolosha V. O., Martin S. L. Polymorphic sequences encoding the first open reading frame protein from LINE-1 ribonucleoprotein particles. J Biol Chem. 1995 Feb 10;270(6):2868–2873. doi: 10.1074/jbc.270.6.2868. [DOI] [PubMed] [Google Scholar]
- Leung P. C., Armstrong D. T. Interactions of steroids and gonadotropins in the control of steroidogenesis in the ovarian follicle. Annu Rev Physiol. 1980;42:71–82. doi: 10.1146/annurev.ph.42.030180.000443. [DOI] [PubMed] [Google Scholar]
- Loeb D. D., Padgett R. W., Hardies S. C., Shehee W. R., Comer M. B., Edgell M. H., Hutchison C. A., 3rd The sequence of a large L1Md element reveals a tandemly repeated 5' end and several features found in retrotransposons. Mol Cell Biol. 1986 Jan;6(1):168–182. doi: 10.1128/mcb.6.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. L., Branciforte D. Synchronous expression of LINE-1 RNA and protein in mouse embryonal carcinoma cells. Mol Cell Biol. 1993 Sep;13(9):5383–5392. doi: 10.1128/mcb.13.9.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. L. LINEs. Curr Opin Genet Dev. 1991 Dec;1(4):505–508. doi: 10.1016/s0959-437x(05)80199-6. [DOI] [PubMed] [Google Scholar]
- Martin S. L. Ribonucleoprotein particles with LINE-1 RNA in mouse embryonal carcinoma cells. Mol Cell Biol. 1991 Sep;11(9):4804–4807. doi: 10.1128/mcb.11.9.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias S. L., Scott A. F., Kazazian H. H., Jr, Boeke J. D., Gabriel A. Reverse transcriptase encoded by a human transposable element. Science. 1991 Dec 20;254(5039):1808–1810. doi: 10.1126/science.1722352. [DOI] [PubMed] [Google Scholar]
- Miki Y., Nishisho I., Horii A., Miyoshi Y., Utsunomiya J., Kinzler K. W., Vogelstein B., Nakamura Y. Disruption of the APC gene by a retrotransposal insertion of L1 sequence in a colon cancer. Cancer Res. 1992 Feb 1;52(3):643–645. [PubMed] [Google Scholar]
- Nur I., Pascale E., Furano A. V. The left end of rat L1 (L1Rn, long interspersed repeated) DNA which is a CpG island can function as a promoter. Nucleic Acids Res. 1988 Oct 11;16(19):9233–9251. doi: 10.1093/nar/16.19.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer A. I., Manova K., Bachvarova R. F. A discrete LINE-1 transcript in mouse blastocysts. Dev Biol. 1993 May;157(1):281–283. doi: 10.1006/dbio.1993.1133. [DOI] [PubMed] [Google Scholar]
- Severynse D. M., Hutchison C. A., 3rd, Edgell M. H. Identification of transcriptional regulatory activity within the 5' A-type monomer sequence of the mouse LINE-1 retroposon. Mamm Genome. 1992;2(1):41–50. doi: 10.1007/BF00570439. [DOI] [PubMed] [Google Scholar]
- Skowronski J., Singer M. F. Expression of a cytoplasmic LINE-1 transcript is regulated in a human teratocarcinoma cell line. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6050–6054. doi: 10.1073/pnas.82.18.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer R. E., Singer M. F., Fanning T. G. Undermethylation of specific LINE-1 sequences in human cells producing a LINE-1-encoded protein. Gene. 1993 Nov 15;133(2):273–277. doi: 10.1016/0378-1119(93)90651-i. [DOI] [PubMed] [Google Scholar]
- van Weerden W. M., Bierings H. G., van Steenbrugge G. J., de Jong F. H., Schröder F. H. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci. 1992;50(12):857–861. doi: 10.1016/0024-3205(92)90204-3. [DOI] [PubMed] [Google Scholar]