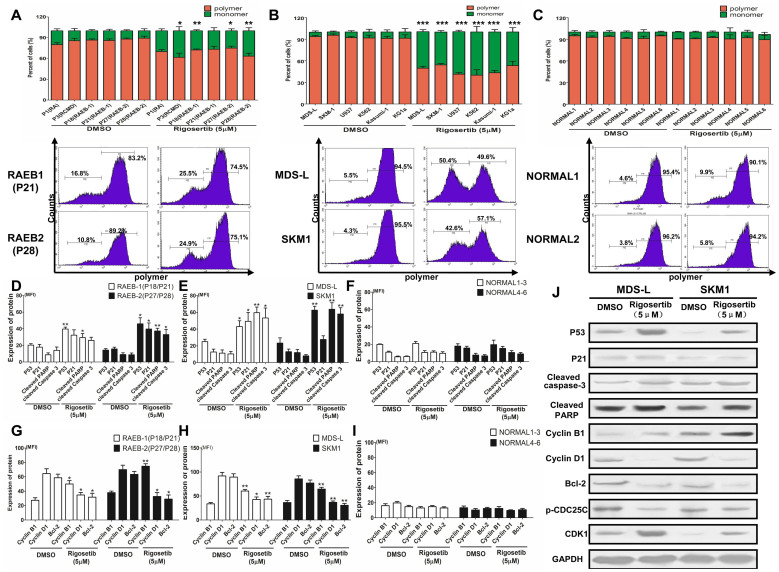
Figure 3. Rigosertib activates the P53-mediated mitochondrial cell death and cycle pathway.
(A) Rigosertib reduced the mitochondrial potentials and produced obvious monomers in MDS CD34+ cells. The representative graphs are shown (down); (B) rigosertib also markedly decreased the mitochondrial potentials in MDS and leukemia cell lines. The representative graphs are shown (down); (C) rigosertib had little impact on the mitochondrial potentials in normal CD34+ cells. The representative graphics are shown (down); Rigosertib markedly increased the expression of P53 pathway-associated apoptosis proteins, such as P53, P21, cleaved PARP and cleaved caspase 3 in primary MDS CD34+ cells (D) and MDS cell lines (E); (F) rigosertib could not increase the level of these apoptosis proteins in normal CD34+ cells; (G) and (H) rigosertib inhibited the expression of the anti-apoptosis proteins bcl-2 and Cyclin D1 in MDS CD34+ cells and cell lines; (I) Rigosertib did not affect these anti-apoptosis proteins in normal CD34+ cells; (J) These P53 pathway-associated proteins were validated via western blot in MDS and leukemia cell lines. The same results were observed for the western blot assay. In addition, rigosertib increased the levels of CDK1 and Cyclin B1 but decreased CDC25C phosphorylation, which may be responsible for G2/M arrest. For each patient or cell line, experiments were carried out three times. For RAEB-1 or RAEB-2 such Figure G, the results included those from two RAEB-1 patients (P18 and P21) and two RAEB-2 patients (P27 and P28). Error bars throughout represent the SEM. *, P<0.05; **, P<0.01; ***, P<0.001 (two-tailed, student T test).