Figure 5.
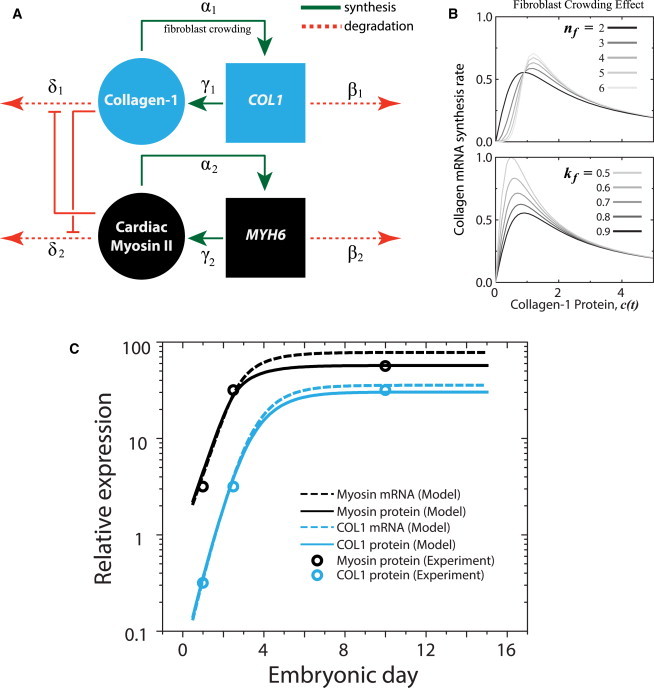
Cardiomyocytes and fibroblasts balance contraction and matrix production during heart development. (A) Assumptions in matrix stiffness limiting fibroblast population α1 (and hence, collagen expression), while encouraging myofibril organization in cardiomyocytes (and hence, myosin expression) are incorporated in a model where tension derived from each module inhibits degradation of the other. (B) Fibroblast crowding is modeled by a Hill function of collagen protein (and hence tension) (13) with nf and kf as the critical point and amplitude, respectively, of the collagen mRNA synthesis rate. (C) Experimentally measured changes in both collagen-1 (COL1) and cardiac myosin expression (circles) in a developing embryonic chick heart (30) can be recapitulated by the model (protein, lines; mRNA, dashed lines). To see this figure in color, go online.
