Abstract
The aim of this study was to investigate the effects of methionine on cell proliferation, antioxidant activity, apoptosis, the expression levels of related genes (HSF-1, HSP70, Bax and Bcl-2) and the expression levels of protein (HSP70) in mammary epithelial cells, after heat treatment. Methionine (60 mg/L) increased the viability and attenuated morphological damage in hyperthermia-treated bovine mammary epithelial cells (BMECs). Additionally, methionine significantly reduced lactate dehydrogenase leakage, malondialdehyde formation, nitric oxide, and nitric oxide synthase activity. Superoxide dismutase, catalase, and glutathione peroxidase enzymatic activity was increased significantly in the presence of methionine. Bovine mammary epithelial cells also exhibited a certain amount of HSP70 reserve after methionine pretreatment for 24 h, and the expression level of the HSP70 gene and protein further increased with incubation at 42 °C for 30 min. Compared to the control, the expression of HSF-1 mRNA increased, and there was a significantly reduced expression of Bax/Bcl-2 mRNA and a reduced activity of caspase-3 against heat stress. Methionine also increased survival and decreased early apoptosis of hyperthermia-treated BMECs. Thus, methionine has cytoprotective effects on hyperthermia-induced damage in BMECs.
Keywords: Methionine, Hyperthermia, Bovine mammary epithelial cells, Oxidative damage, Heat shock protein 70, Apoptosis
Introduction
Under heat stress conditions in the tropics and subtropics during the summer, lactating dairy cows suffer from reduced food intake and lactation performance (Liu 2004; Chang 2003; Wang and Liu 2008). Heat stress induces a variety of physiological and biochemical reactions in dairy cows, reduces food intake and performance traits, reduces breeding performance and immunity, and causes serious harm to dairy production, thus affecting the economic benefits of dairy cattle producers (Yan and Li 2004; Du et al. 2006).
Lactoprotein is an important indicator of the quality of milk and is synthesized on the ribosomes of the rough endoplasmic reticulum in mammary epithelial cells. Therefore, the number of mammary epithelial cells largely determines the protein synthesis of lactoprotein (Mercier and Vilott 1993; Guo 2007; Zhou 2010). During heat stress, transcription and translation of RNA are inhibited, as is cell cycling growth; membrane permeability is also changed (Sonn et al. 2002). Heat stress induces many abnormalities in cell function, including the inhibition of protein synthesis, changes in protein folding and function, and changes in metabolism and membrane fluidity, all of which reduce proliferation (Collier et al. 2008). This study showed that 42 °C is a turning point for thermal tolerance, and when temperatures were higher than 42 °C, the cell’s own regulatory role was gradually reduced. As the temperature rose to 46 °C, a number of key proteins in the cells were denatured (Lepock 2005). Heat shock protein (HSP) integrated the mutability of the lactoprotein in the form of molecular chaperones, prevented the irreversible aggregation of the protein, and enhanced the ability to resist the heat stress of cells (Kampinga 2006). Expression of the HSP genes induced by heat stress was adjusted by the heat shock transcription factor 1 (HSF-1). In normal conditions, a certain amount of HSP70 exists in the cell, and the HSF-1 monomers without DNA-binding activity in the cytoplasm form a complex with HSP70 (Wu 1995; Sun et al. 2007; Beere and Green 2001). In conditions of heat stress, an external signal stimulates the activation of HSF-1 and combines with the heat shock element (HSE) to induce expression of the HSP (Morano and Thiele 1999; Lindquist and Craig 1988).
Mitochondria are the “power plants” of cells and are the main location of intracellular oxidative phosphorylation and ATP synthesis. Increasingly, studies have found that mitochondria play a pivotal role in apoptosis, and many apoptotic signals converge on mitochondria to activate or inhibit apoptosis (Halestrap et al. 2002; Hengartner 2000).
The Bcl-2 family includes transmembrane proteins located on the mitochondria that may synergize with other transmembrane proteins to affect the regulation of mitochondrial membrane permeability and membrane potential that could play a “master switch” role in apoptosis (Chipuk et al. 2010; Patel et al. 2009). The Bcl-2 family consists of two categories, namely one includes the anti-apoptotic factors, such as Bcl-2, Bcl-xL, Mcl-1, etc. and can function in the closing activity of pore formation by Bax, preventing the release of the apoptotic factor cytochrome C by the mitochondria and blocking apoptosis or preventing the release of Ca2+ and AIF, blocking the caspase cascade and inhibiting apoptosis; the other includes pro-apoptotic factors, such as Bax, Bcl-xS, Bad, Bak, etc. and can be inserted into the outer mitochondrial membrane forming channels, releasing the active substances of apoptosis (Gross et al. 1999; Nutt et al. 2002; Gao and Suchida 1999). These two proteins interact via a dipolymer network, where the balance between the different dipolymers determines cell apoptosis so that the higher the ratio of Bax/Bcl, the higher the incidence of apoptosis (Chang et al. 2005).
Methionine is one of the major limiting amino acids for dairy cows (Han 2009; Lobley et al. 1987; Girard et al. 2005). The benefits of methionine supplementation include improvement in milk production and antioxidant capacity, reduction in lymphocyte apoptosis, promotion of the expression of the Bcl-2 genes of the lymphocytes, and inhibition of the Bax gene (Han 2009; Nichols et al. 1998). Methionine can increase the synthesis of lactoprotein by the extracorporeal perfusion of free amino acids in lactating goats (Lin et al. 2009). In vitro tests on bovine mammary cells show that adding a certain amount of free lysine and methionine can improve the expression of αs1 casein (Li 2010; Wang 2008). Because of its potential role in promoting cell proliferation in vitro, methionine affects the metabolic capacity of the cell and promotes cell division (Breillout et al. 1990; Wu et al. 2004).
The present study was performed to investigate the effects of methionine on mammary epithelial cells after heat treatment. We measured the cell proliferation, antioxidant activity, apoptosis, and expression of related genes and proteins in response to high temperatures in order to provide a theoretical basis for the study of bovine mammary epithelial cells against heat stress, and we investigated the protective effects of methionine for heat-stressed cows and their udders at the cellular level.
Materials and methods
Cell culture
Mammary tissues were obtained from a healthy Holstein dairy cow in the middle stage of lactation. In vitro cultures of mammary epithelial cells were prepared in accordance with the established methods of the Nanjing Agricultural University of Dairy Cow Science Institute laboratory (Du et al. 2007). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 4.5 mg/ml glucose and supplemented with 10 % fetal bovine serum (Life Technologies, Gaithersburg, MD, USA), 1 % antibiotic-antimycotic solution (Sigma-Aldrich, St. Louis, MO, USA), and 2 mmol/L glutamine (Sigma-Aldrich, St. Louis, MO, USA) in a humidified incubator, with 5 % CO2 at 37 °C. After the cells reached 80 % confluence, they were removed with 0.25 % trypsin and 0.15 % trypsin plus 0.02 % EDTA. Positive staining of cytokeratin-18 was conducted in the epithelial cells by the immunocytochemical staining method.
Hyperthermia treatment of cultured cells
The dispersed cells were plated at a concentration of 5 × 104 cells/ml in 6-well flat-bottomed plates, with the medium supplemented in the absence (control) and presence of methionine (0, 30, and 60 mg/L). After 24 h of culturing at 37 °C in a water-saturated atmosphere, heat shock was performed by immersion in a water bath, pre-heated in advance, to achieve a stable temperature of 42 °C. The growth media was replaced with media warmed to 42 °C, and the plates or flasks were sealed with parafilm and immersed for 0.5 h. The media was then replaced with growth media at 37 °C and incubated under standard growing conditions for 6 h.
MTT cell viability assay
The cells were treated as described above for the hyperthermia treatment. After 24 h, the medium was removed and 200 μl MTT medium (0.5 mg/ml MTT reagent in fresh medium) was added to each well. After incubation for 4 h, the MTT reagent was removed and 150 μl of dimethyl sulfoxide (Jiancheng Bioengineering Institute, Nanjing, China) was added to each well, followed by 10 min of gentle shaking. Cell viability was assessed by a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide trypan blue exclusion assay as the proportion of absorbance values to the control. The absorbance was read at a wavelength of 570 nm in a MULTISKAN MK3 (Thermo Electron Corporation, USA) as the value expressing the entity of proliferation.
Analysis of BMEC apoptosis with flow cytometry
A hanging liquid basal medium was used for the preparation of the bovine mammary epithelial cells (BMECs) cultured for 3 to 5 days. After 2 days of culturing at 37 °C in 6-well culture plates, the cells were treated with different concentrations of methionine (0, 30, and 60 mg/L), and hyperthermia treatment was given as above. Each treatment was repeated three times. The cells in each group were collected, and apoptosis was assayed by an annexin V/PI double staining kit (BD Pharmingen, CAT: 556547).
Determination of free radicals
The level of lipid peroxidation was determined by measuring the concentration of malondialdehyde (MDA) following the method of Esterbauer and Cheeseman (Esterbauer and Cheeseman 1990), and the MDA level was determined by 2-thiobarbituric acid reactive substance (TBARS) chromometry (Zhang et al. 2011). The reaction of nitric oxide (NO) with oxygen and water to form nitrate and nitrite, which react with nitrate’s chromogenic reagent, can generate light red azo compounds that indirectly detect the concentration of NO by colorimetric analysis. Nitric oxide synthase (NOS) catalyzed the l-Arg reactant with molecular oxygen to generate NO, the NO reaction with the nucleophilic substance formed a colored compound, and NOS activity was assayed by absorbance changes at 530 nm. Cytotoxicity was estimated by the quantification of lactate dehydrogenase (LDH) activity in the culture medium versus the total LDH activity in the samples after treatment.
The cultured cells were treated with 10 % Triton X-100, and the LDH activity was assayed by absorbance changes at 440 nm with an LDH kit. Commercial assay kits for MDA, NO, NOS, and LDH were provided by the Nanjing Jiancheng Biotechnology Research Institute (Nanjing, China), and the measurements were performed according to the protocol provided by the manufacturer in the laboratory of the Nanjing Agricultural University of Dairy Cow Science Institute.
Determination of antioxidant enzyme activity
Adding enzyme-specific substrates to the cell homogenate and measuring the rates of the disappearance of the substrate by spectrophotometry determined the activities of the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px). The SOD activity in the homogenate was assayed by the inhibition at 25 °C of pyrogallol autoxidation by SOD (with and without sample) and was followed kinetically at 550 nm (Jiancheng Bioengineering Institute, Nanjing, China). One unit of SOD is defined as the amount of enzyme that causes 50 % inhibition of pyrogallol autoxidation. The production of a superoxide radical was determined by using a xanthine/xanthine-oxidase system to reduce cytochrome C, and it was calculated as units per milligram protein × minutes. The CAT activity (Aebi 1984) was calculated as the reduction of H2O2 in nanomoles per milligram protein × minutes. The GSH-Px activity was measured by using H2O2 as a substrate by applying the method of Rotruck et al. (1973).
Quantitative real-time PCR
The total RNA was isolated by using the AxyPrep™ Multisource Total RNA Miniprep kit (Axygen, USA), and an equivalent amount of RNA was converted into complementary DNA (cDNA) with the PrimeScript™ RT reagent kit (Takara, Japan). Subsequently, real-time PCR was performed using an ABI 7500 Sequencing Detection System (Applied Biosystems, USA) and SYBR® Premix Ex Taq™ II (Takara, Japan). All of the procedures were performed according to the manufacturer’s protocols. The PCR conditions included a primary denaturation step at 95 °C for 20 s followed by 40 cycles of 2 min at 95 °C, 20 s at a corresponding annealing temperature of each primer pair, and 1 min at 72 °C. The sequences of the specific primer pairs for HSF-1, HSP70, Bax, Bcl-2, and GAPDH are presented in Table 1. The comparative 2−△△CT method was used to calculate the relative expression level of each targeted gene, with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) serving as the housekeeping gene. RT-PCR data were normalized by measuring average cycle threshold (Ct) ratios between candidate genes and the control gene, GAPDH.
Table 1.
Nucleotide sequence of specific primer pairs applied for the quantitative real-time PCR detection of heat shock factor-1 (HSF-1), heat shock protein-70 (HSP70), Bax, Bcl-2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
| Gene | Primer pairs and probes | Product length (bp) | Annealing (°C) |
|---|---|---|---|
| HSF-1 | Forward: 5′-TCTGACGGCAACTTCAACTG-3′ Reverse: 5′-TCGAAGGAAGTCCAATGTCC-3′ |
166 | 60 |
| HSP70 | Forward: 5′-CAAGATCACCATCACCAACG-3′ Reverse: 5′-TTGTACTTTTCCGCCTCCTG-3′ |
259 | 60 |
| Bax | Forward: 5′-TCTGACGGCAACTTCAACTG-3′ Reverse: 5′-TCGAAGGAAGTCCAATGTCC-3′ |
250 | 60 |
| Bcl-2 | Forward: 5′-ATGTGTGTGGAGAGCGTCAA-3′ Reverse: 5′-CCTTCAGAGACAGCCAGGAG-3′ |
136 | 60 |
| GAPDH | Forward: 5′-GGGTCATCATCTCTGCACCT-3′ Reverse: 5′-GGTCATAAGTCCCTCCACGA-3′ |
378 | 60 |
Western blot analysis
The cultured BMECs were washed twice with PBS, harvested with 1 ml of ice-cold RIPA lysis buffer, and incubated for 1 h at 4 °C. The insoluble material was removed by centrifugation at 10,000 g for 10 min at 4 °C. The protein content was determined by the Folin-phenol method with BSA as a standard substance, and the proteins were then placed in a diluted SDS sample buffer and denatured for 5 min at 99 °C, before being subjected to 12.5 % SDS-PAGE. Sixty micrograms of total protein was loaded in each lane, subjected to electrophoresis, and subsequently transferred to PVDF membranes (Millipore Corp, Bedford, MA, USA) by electroblotting. The membranes were blocked with 5 % fat-free milk at room temperature for 1 h and then incubated with primary antibody (1:1,000 for rabbit monoclonal anti-HSP70; 1:2,000 for rabbit monoclonal GAPDH, catalog number: 10995-1-AP, supplier: Pierce) at 4 °C overnight; after three washes in T-TBS, the membranes were incubated with anti-rabbit IgG for 1 h at room temperature. After washing, the signals were visualized using enhanced chemiluminescence (ECL) and X-ray film (Kodak, USA), with GAPDH serving as an internal control. The results of the representative chemiluminescence were scanned and densitometrically analyzed using the ImageMaster VDS system (Amersham, UK), with the help of the ImageQuant TL site program.
Apoptosis assay via measurement of caspase-3 activity
The relative caspase-3 activity was determined using a caspase-3 assay kit according to the manufacturer’s instructions (Jiancheng Bioengineering Institute, Nanjing, China). This assay is based on the generation of free p-nitroanilide (pNA) chromophores following the cleavage of the acetyl-Asp-Glu-Val-Asp (DEVD)-pNA substrate by caspase-3 and subsequent absorbance reading at 405 nm. Briefly, the BMECs were placed in 24-well plates at a density of 2 × 106 cells/well and incubated at 37 °C in a 5 % CO2 atmosphere. Following the treatment of the cells with different concentrations of methionine (0, 30, and 60 mg/L), the hyperthermia treatment was given as above. The cultured BMECs were washed with PBS, trypsinized, harvested in a lysis buffer, and centrifuged to eliminate cellular debris. Fifty-microliter aliquots of the cell extracts were subjected to caspase-3 measurements according to the manufacturer’s protocol, and the absorbance was read at 405 nm using a MULTISKAN MK3 (Thermo Electron Corporation, USA).
Statistical analysis
The data were expressed as the mean ± SD and analyzed by ANOVA and Duncan’s multiple range tests using the SPSS 17.0 software system. p < 0.05 was considered to be significant.
Results
Morphological identification of BMEC
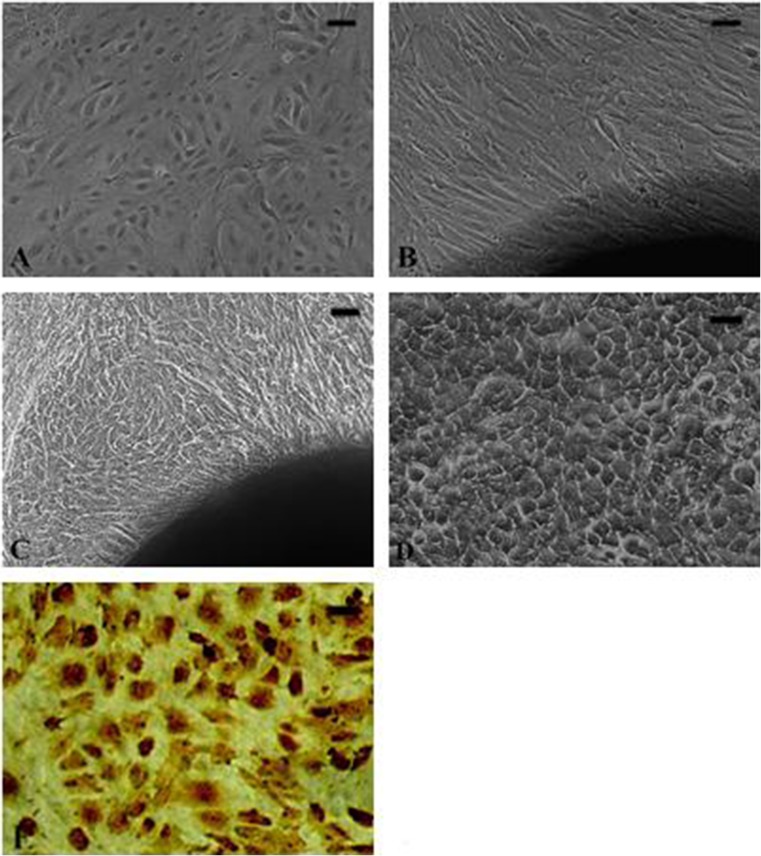
Fibroblasts have a long fusiform morphology, while mammary epithelial cells are flat and polygonal, with a circular nucleus near the center of the cytoplasm as a typical characteristic. Cells grown very closely form a mesh or network, which is also characteristic of epithelial cells (Fig. 1a–d). Positive staining of cytokeratin-18 was done in the epithelial cells via an immunocytochemical staining method, and 95 % of the cells were positive (Fig. 1e). The mesh structure may be related to the secretion of the hyaluronidase of the stromal cells, inducing liquefaction that causes the cells to separate from each other and fall off the plate.
Fig. 1.
Cultured cells. a Bovine epithelial mammary cells. b Fibroblast emerging from the tissue edges. c Polygon-like aggregates of BMECs emerging from the tissue edges. d Typical net-balloon construction of the epithelial cells. e Positive staining of cytokeratin-18 in the epithelial cells by immunocytochemical staining methods. Scale bar = 20 um
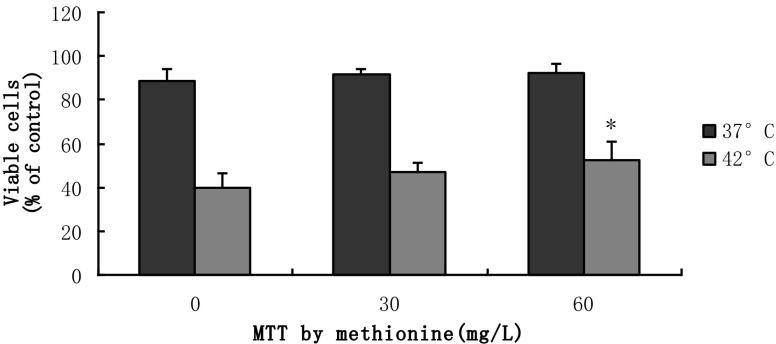
Effect of methionine on cell viability
The treatment with different concentrations of methionine increased the cell viability as determined by an MTT assay (Fig. 2). The viability of the 37 °C controls was significantly higher than the 42 °C group (p < 0.01). Addition of 60 mg/mL of methionine provided the highest level of cell viability. The cell viability following the 60 mg/L methionine treatment was significantly improved by 33 %, when compared to the 0 mg/L control in the hyperthermia-treated BMECs (p < 0.05).
Fig. 2.
Cytoprotection of methionine in heat-treated and non-heat-treated BMECs. The cell viability was quantified via MTT assay and expressed as the proportion of absorbance values to the control (*p < 0.05 vs. 0 mg/L at 42 °C. Values are expressed as the mean ± SD of two independent experiments; n = 8)
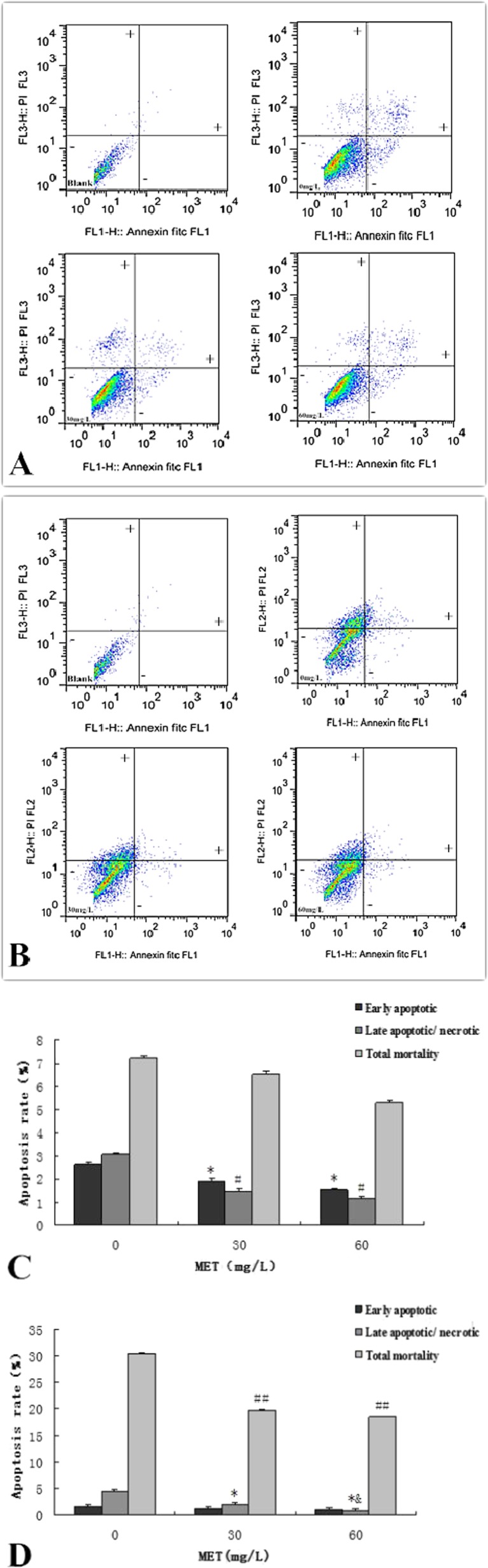
Effects of methionine on apoptosis of BMEC
Annexin V and propidium iodide (PI) double staining divided the cells into three categories: living cells (annexin V and PI negative), early apoptotic cells (annexin V positive, PI negative), and late apoptotic/necrotic cells (annexin V and PI positive) (Fig. 3a, b). The results showed that after adding different concentrations of methionine to the 37 °C group, the rate of early apoptosis, late apoptosis/necrosis cells was significantly decreased when compared with the control group (p < 0.05), but the differences between the treatment groups were not significant (Fig. 3c). The influence of high temperature on the mammary epithelial cell apoptosis and late apoptosis rates was comparatively large (Fig. 3d). Adding methionine at 30 and 60 mg/L reduced the late apoptosis by 57 and 80 %, respectively (p < 0.05), while the total mortality was reduced by 35 and 39 % when compared to 0 mg/L (p < 0.01) under hyperthermia.
Fig. 3.
Effects of methionine on apoptosis on BMECs. a Effects of methionine on apoptosis of non-heat-treated BMECs by annexin V and PI double staining. With the annexin V and PI double staining labeled by FITC, the cells were divided into three quadrants as follows: living cells (FITC-, PI-); early apoptotic cells (FITC+, PI-); late apoptotic/necrotic cells (FITC+, PI+). b Effects of methionine on apoptosis of hyperthermia-treated BMECs by annexin V and PI double staining. Labeling as in a. c Statistical chart of apoptosis rate against 37 °C. (*p < 0.05 vs. 0 mg/L early apoptotic; # p < 0.05 vs. 0 mg/L late apoptotic/necrotic). Values are expressed as the mean ± SD of three independent experiments; n = 8. d Statistical chart of apoptosis rate against 42 °C. (*p < 0.05 vs. 0 mg/L late apoptotic/necrotic; &p < 0.05 vs. 30 mg/L late apoptotic/necrotic, ## p < 0.01 vs. 0 mg/L total mortality. Values are expressed as the mean ± SD of three independent experiments; n = 8)
Effects of methionine on free radicals of BMEC
Before heat stress, the MDA formation at 60 mg/L methionine was the lowest and significantly lower than the 0 mg/L control group (p < 0.05). The MDA formation was reduced by 31 % at 60 mg/L of methionine in the hyperthermia-treated BMECs, when compared to 0 mg/L (p < 0.01; Table 2).
Table 2.
Effects of methionine on malondialdehyde (MDA) formation
| Methionine group | 0 mg/L | 30 mg/L | 60 mg/L |
|---|---|---|---|
| 37 °C group | 2.12 ± 0.06 | 1.93 ± 0.09 | 1.72 ± 0.09* |
| 42 °C group | 2.78 ± 0.10 | 2.29 ± 0.11# | 1.93 ± 0.14## |
*p < 0.05 vs. 0 mg/L 37 °C; # p < 0.05 vs. 0 mg/L 42 °C, ## p < 0.01 vs. 0 mg/L 42 °C. Values are mean ± SD, n = 8, nmol/mL
The data represented by Table 3 show that at 60 mg/L, the content of NO was at a minimum, but was not significantly different from the other methionine treatment groups (p > 0.05). After heat stress, the content of the NO was significantly increased, and adding methionine at 60 mg/L reduced it by 23 % when compared with the 0 mg/L control group of hyperthermia-treated BMECs (p < 0.01).
Table 3.
Effects of methionine on nitric oxide (NO) formation
| Methionine group | 0 mg/L | 30 mg/L | 60 mg/L |
|---|---|---|---|
| 37 °C group | 3.52 ± 0.52 | 3.30 ± 0.16 | 2.94 ± 0.44 |
| 42 °C group | 4.38 ± 0.40 | 3.69 ± 0.52 | 3.36 ± 0.29**# |
**p < 0.01 vs. 0 mg/L 42 °C; #p < 0.05 vs. 30 mg/L 42 °C. Values are mean ± SD, n = 8, umol/g protein
After adding the methionine, the activity of the NOS was reduced. Before heat stress, the NOS activity at 60 mg/L of methionine was the lowest, at 17 % lower than the 0 mg/L control group (p < 0.05). After heat stress, the activity of the NOS was reduced by 35 and 43 % after treatment with 30 and 60 mg/L of methionine (p < 0.01), but the differences between the treatment groups were not significant (p > 0.05; Table 4).
Table 4.
Effects of methionine on nitric oxide synthase (NOS) activity
| Methionine group | 0 mg/L | 30 mg/L | 60 mg/L |
|---|---|---|---|
| 37 °C group | 0.82 ± 0.03 | 0.79 ± 0.11 | 0.68 ± 0.03* |
| 42 °C group | 1.75 ± 0.29 | 1.14 ± 0.16## | 0.99 ± 0.11## |
*p < 0.05 vs. 0 mg/L 37 °C; ## p < 0.01 vs. 0 mg/L 42 °C. Values are mean ± SD, n = 8, U/mL
In the hyperthermia-treated BMECs, the activity of the LDH was higher than in the non-heat-treated group. The activity of the LDH at 60 mg/L of methionine was reduced by 21 % under non-hyperthermia conditions and by 17 % under hyperthermia conditions, when compared with the 0 mg/L control group (p < 0.05), but the differences between the treatment groups were not significant (p > 0.05; Table 5).
Table 5.
Effects of methionine on lactated dehydrogenase (LDH) leakage
| Methionine group | 0 mg/L | 30 mg/L | 60 mg/L |
|---|---|---|---|
| 37 °C group | 2631.68 ± 413.40 | 2566.19 ± 276.69 | 2080.13 ± 249.02* |
| 42 °C group | 3442.69 ± 301.65 | 3116.51 ± 340.71 | 2840.42 ± 291.29# |
*p < 0.05 vs. 0 mg/L 37 °C; # p < 0.05 vs. 0 mg/L 42 °C. Values are mean ± SD, n = 8, U/mL
Effects of methionine on antioxidant enzyme activities in BMEC
The SOD activity of the 37 °C controls was higher than in the 42 °C group (Table 6). In the non-heat-treated hyperthermia-treated BMECs, the SOD activity of the 60 mg/L methionine group was significantly higher than in the other groups, while the rest of the groups were not significant (p > 0.05). Adding methionine at 60 mg/L markedly increased the SOD activity of the hyperthermia-treated BMECs, which was 18 % higher than in the 0 mg/L control group (p < 0.05).
Table 6.
Effects of methionine on superoxide dismutase (SOD) activity
| Methionine group | 0 mg/L | 30 mg/L | 60 mg/L |
|---|---|---|---|
| 37 °C group | 24.73 ± 2.17 | 25.22 ± 0.63 | 26.62 ± 1.03 |
| 42 °C group | 11.76 ± 1.22 | 12.70 ± 0.89 | 13.92 ± 0.73* |
*p < 0.05 vs. 0 mg/L 42 °C. Values are mean ± SD, n = 8, U/mL
Before heat stress, the CAT activity gradually increased after adding different concentrations of methionine. The CAT activity of the 60 mg/L methionine group was the highest, while the rest of the groups were not significant (p > 0.05). After heat treatment, the CAT activity was reduced; adding methionine at 30 and 60 mg/L markedly increased the CAT activity by 48 and 58 %, which was significantly different at 0 mg/L (p < 0.05; Table 7).
Table 7.
Effects of methionine on catalase (CAT) activity
| Methionine group | 0 mg/L | 30 mg/L | 60 mg/L |
|---|---|---|---|
| 37 °C group | 2.27 ± 0.19 | 2.35 ± 0.37 | 2.67 ± 0.36 |
| 42 °C group | 1.42 ± 0.34 | 2.10 ± 0.46* | 2.25 ± 0.46* |
*p < 0.05 vs. 0 mg/L 42 °C. Values are mean ± SD, n = 8, U/mL
After adding the methionine, the GSH-Px enzyme activities of the non-heat-treated group were higher than those of the heat stress group. In the non-heat-treated hyperthermia-treated BMECs, the GSH-Px enzyme activities were increased by 3.7 and 5.1 % after treatment with 30 and 60 mg/L of methionine (p < 0.05). After heat treatment, the enzyme activity of the GSH-Px at 60 mg/L of methionine was 15 % higher than in the 0 mg/L control group (p < 0.01), but the differences between the treatment groups were not significant (p > 0.05; Table 8).
Table 8.
Effects of methionine on glutathione peroxidase (GSH-Px) enzymatic activity
| Methionine group | 0 mg/L | 30 mg/L | 60 mg/L |
|---|---|---|---|
| 37 °C group | 84.82 ± 0.69 | 87.98 ± 1.21* | 89.13 ± 0.71* |
| 42 °C group | 69.31 ± 1.45 | 75.77 ± 1.56# | 79.46 ± 1.73## |
*p < 0.05 vs. 0 mg/L 37 °C; # p < 0.05 vs. 0 mg/L 42 °C, ## p < 0.01 vs. 0 mg/L 42 °C. Values are mean ± SD, n = 8, GSH-Px activity unit (per milligram protein per minute oxidate 1 μmol GSH is defined as an activity unit)
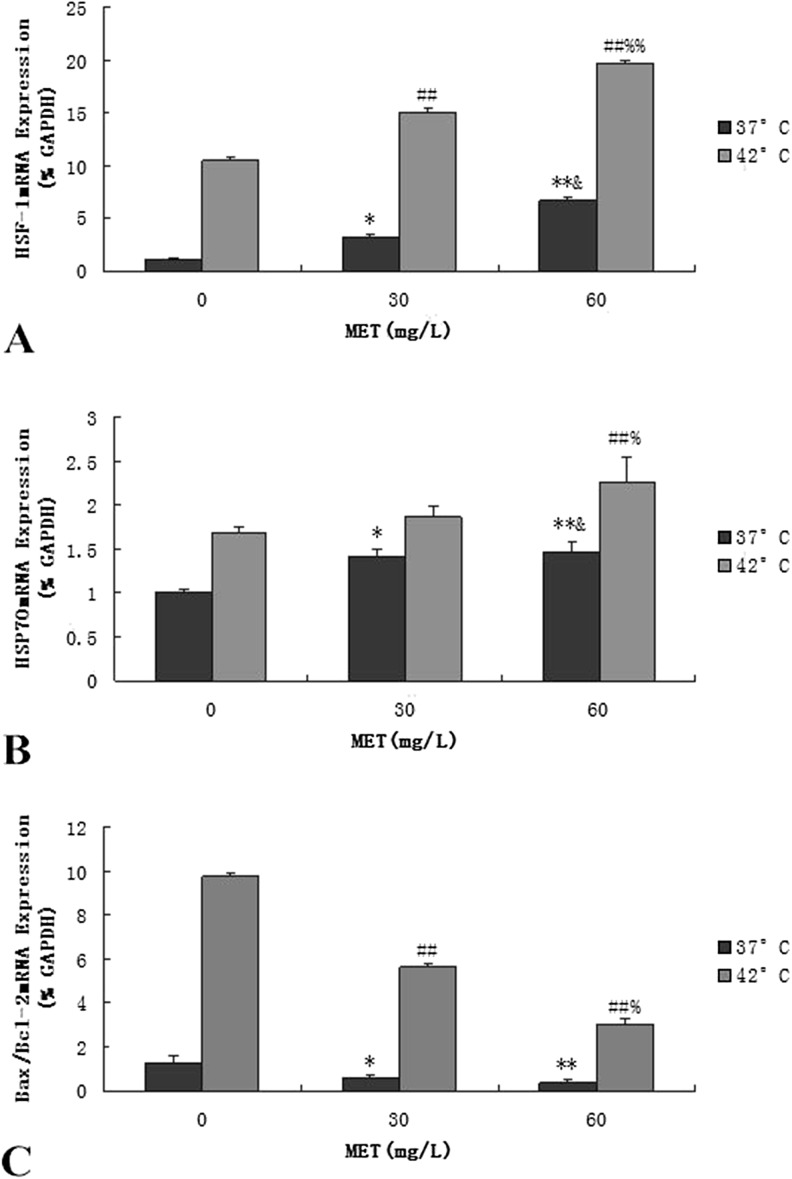
Effects of methionine on the mRNA levels of the HSF-1, HSP70, and Bax/Bcl-2 genes in BMEC
The HSF-1 mRNA expression was determined by real-time PCR, and the optical density of the HSF-1 was corrected for that of the GAPDH. The results show that methionine can induce the expression of HSF-1 mRNA, increasing it in a dose-dependent manner (Fig. 4a). In the 37 °C treatment groups, to which methionine was added at 60 mg/L, the expression of the HSF-1 mRNA was significantly higher than in the 30 mg/L control group (p < 0.05) and significantly different at 0 mg/L (p < 0.01). After heat stress, the HSF-1 mRNA expression at 60 mg/L of methionine was the highest and significantly improved by 88 and 31 % when compared to the 0 and 60 mg/L groups, respectively (p < 0.01).
Fig. 4.
Effects of methionine on the mRNA expression on BMECs. a Effects of methionine on the mRNA expression of the HSF-1 gene in BMECs. (*p < 0.05, **p < 0.01 vs. 0 mg/L 37 °C; &p < 0.05 vs. 30 mg/L 37 °C; ## p < 0.01 vs. 0 mg/L 42 °C; %% p < 0.01 vs. 30 mg/L 42 °C. Values are expressed as the mean ± SD of two independent experiments; n = 8). b Effects of methionine on the mRNA expression of the HSP70 gene in BMECs. (*p < 0.05, **p < 0.01 vs. 0 mg/L 37 °C; &p < 0.05 vs. 30 mg/L 37 °C; ## p < 0.01 vs. 0 mg/L 42 °C; % p < 0.05 vs. 30 mg/L 42 °C. Values are expressed as the mean ± SD of two independent experiments; n = 8). c Effects of methionine on the mRNA expression of the Bax/Bcl-2 gene in BMECs. (*p < 0.05, **p < 0.01 vs. 0 mg/L 37 °C; ## p < 0.01 vs. 0 mg/L 42 °C; % p < 0.05 vs. 30 mg/L 42 °C. Values are expressed as the mean ± SD of two independent experiments; n = 8)
As shown in Fig. 4b, the expression of the HSP70 mRNA was consistent with the HSF-1 mRNA response. After heat stress, the mRNA expression levels of the HSP70 on each group were significantly increased. Heat stress can increase the HSP70 mRNA transcriptional level of the BMECs, thereby protecting the cells from damage. After adding different concentrations of methionine to the 37 °C group, the expression of the HSP70 mRNA was significantly increased (p < 0.05), and its detection in the 60 mg/L methionine group was 47 % higher than in the 0 mg/L control group (p < 0.01). Adding methionine at 60 mg/L increased the expression of the HSP70 mRNA by 22 %, when compared to the 30 mg/L control group (p < 0.05), and increased it by 35 % compared with the 0 mg/L group (p < 0.01) under hyperthermia.
High temperature induced the mRNA level changes of the Bcl-2 family and may mediate mitochondrial pathway induced apoptosis. In the 37 °C treatment groups, after adding different concentrations of methionine, the expression of Bax/Bcl-2 mRNA was significantly decreased (p < 0.05) and in the 60 mg/L methionine group was detected at greater than 71 % lower than in the 0 mg/L control group (p < 0.01). After 42 °C heat treatment, the expression of Bax/Bcl-2 mRNA was significantly increased. After adding methionine at 60 mg/L, the expression of Bax/Bcl-2 mRNA was decreased by 47 %, when compared to the 30 mg/L control group (p < 0.05), and reduced by 69 % when compared with the 0 mg/L control group (p < 0.01; Fig. 4c).
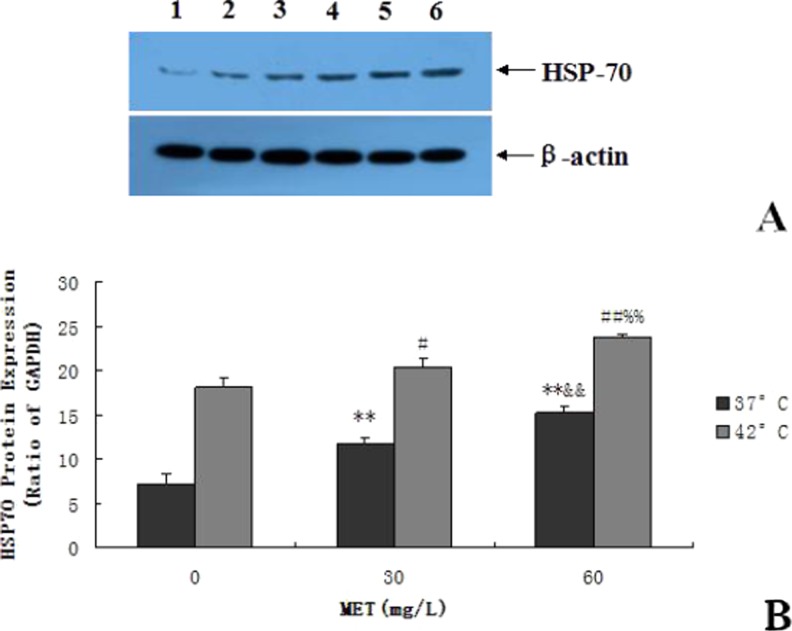
Effects of methionine on the protein levels of Hsp70 in BMEC
As shown in Fig. 5a, b, the protein expression levels of HSP70 in each group were significantly increased after heat stress. After using GELPro Analyzer software to scan the quantitative results, the Western blot of the HSP70 results was consistent with the HSP70 mRNA response to heat stress. Before heat stress, the protein expression of the HSP70 was improved by 62 and 109 % after treatment with 30 and 60 mg/L of methionine (p < 0.01). After heat stress, and with the addition of methionine at 60 mg/L, the protein expression of the HSP70 was significantly improved by 31 and 17 %, when compared to the 0 and 30 mg/L groups, respectively (p < 0.01).
Fig. 5.
Effects of methionine on the protein expression of HSP70 in BMECs. a Detection of the protein expression of HSP70 using Western blot assay. Panels 1 through 3 represent the protein expression of HSP70 after adding different concentrations of methionine (0, 30, and 60 mg/L) in non-heat-treated BMECs. Panels 4 through 6 represent the protein expression of HSP70 after adding different concentrations of methionine (0, 30, and 60 mg/L) in heat-treated BMECs. b Effects of methionine on the protein expression of HSP70 in BMECs. (**p < 0.01 vs. 0 mg/L 37 °C; &&p < 0.01 vs. 30 mg/L 37 °C; # p < 0.05 vs. 0 mg/L 42 °C, ## p < 0.01 vs. 0 mg/L 42 °C; %% p < 0.01 vs. 30 mg/L 42 °C. Values are expressed as the mean ± SD of two independent experiments; n = 8)
Methionine reduces hyperthermia-induced apoptosis in BMEC
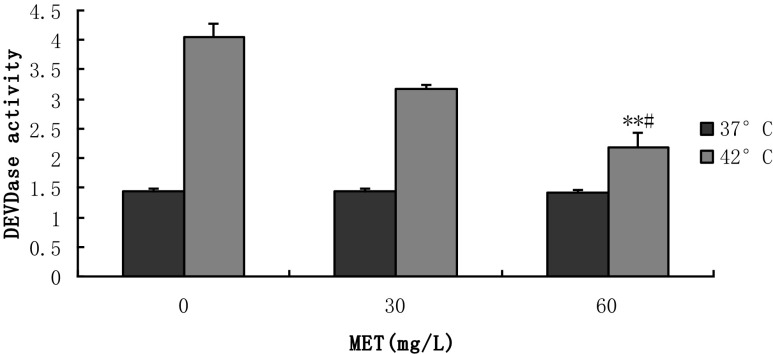
The level of activated caspase-3 is based on the generation of free p-nitroanilide (pNA) chromophores, following the cleavage of the acetyl-Asp-Glu-Val-Asp (DEVD)-pNA substrate by caspase-3. As seen in Fig. 6, the results showed that the level of activated caspase-3 was significantly increased in the hyperthermia-treated BMECs. Heat stress activated the activity of the caspase-3, which was the key factor in the apoptotic cascade reaction, and caused apoptosis in the BMECs. Before heat stress, following the conditions described above, the lowest level of the activated caspase-3 was observed in the 60 mg/L methionine group, while the differences between the treatment groups were not significant (p > 0.05). After adding methionine at 60 mg/L, the level of activated caspase-3 was decreased by 31 % when compared to the 30 mg/L control group (p < 0.05) and reduced by 46 % when compared with the 0 mg/L under hyperthermia (p < 0.01).
Fig. 6.
Detection of apoptotic activity using the caspase-3 assay kit. This assay is based on the generation of free p-nitroanilide (pNA) chromophores following the cleavage of the acetyl-Asp-Glu-Val-Asp (DEVD)-pNA substrate by caspase-3. The activity of caspase-3 can be suppressed by DEVD, and DEVD can be degraded by DEVDase. If DEVDase content is more, it will be explained that DEVD is relatively smaller; thus, the activity of caspase-3 will be relatively large. So the content of DEVDase and the activity of caspase-3 were positively correlated. (**p < 0.01 vs. 0 mg/L 42 °C; # p < 0.05 vs. 30 mg/L 42 °C. Values are expressed as the mean ± SD of two independent experiments; n = 8)
Discussion
The structure of the BMEC membrane was severely damaged by the hyperthermia treatment. Condensed nuclei and vacuolated cytoplasm occurred, many cell pieces were released into the medium, and cytolysis and disorganization of the cells were found (Hong 2010). High temperatures can inhibit the growth of cells; change the structure and function of proteins, membrane permeability, and metabolism; and eventually inhibit proliferation (Sonn et al. 2002; Collier et al. 2008).
When added as an exogenous nutrient, methionine plays a role in cell proliferation. Adding exogenous methionine in the DMEM medium without fetal bovine serum can increase the proliferation rate of normal healthy adult lymphocytes in vitro (Crott et al. 2001). In addition, other studies suggest that adding 57 mg/L (0.382 mmol/L) of methionine can significantly increase the expression of the asl-casein protein gene, which provides a basis for further research into methionine in the milk protein synthesis mechanisms of in vitro BMECs (Liu et al. 2007).
Research data are consistent with reports that a reduced trend in thermal stress levels follows the addition of methionine, up to a certain value (Mu et al. 2014). Methionine supplementation at 30 and 60 mg/L could block these deleterious effects through the reduction of MDA and an increase in SOD, thereby increasing survival and decreasing early apoptosis. But the differences between treatment groups (30 and 60 mg/L) was not significant, so this test selected 30 and 60 mg/L of methionine for further experiments to determine which has the best treatment effect on BMECs.
This experimental study was on the effects of methionine on BMECs; the results show that adding methionine at 60 mg/L markedly rescued the proliferation of heat-treated BMECs (p < 0.05). These data are consistent with reports that methionine increases the reproductive and lactation performances in dairy cows (Nichols et al. 1998; Wang 2012; Yang 2007; Liu et al. 2007) and alleviates the effects of heat stress (Han 2009). Thus, our in vitro data are consistent with these findings.
The structure of the BMEC membrane was severely damaged by hyperthermia treatment. The mitochondrial electronic chain and energy production had been switched off, membrane permeability increased, and transmembrane potential was lowered (Koremer et al. 1997), which can precipitate apoptosis. Early apoptotic cell membranes were intact, while the necrotic cell membrane integrity was destroyed at an early time period (Wang 2007). In the current study, our results agreed with the finding that adding methionine at 60 mg/L markedly decreased early apoptosis, while it significantly decreased late apoptosis (p < 0.05) and total mortality (p < 0.01) of hyperthermia-treated BMECs. Morphological damage induced by hyperthermia can be significantly attenuated, as indicated by the relative integral of the cell membranes, increasing the survival rate from oxidative injury and reducing apoptosis in BMECs (Han 2009).
Cells produce large amounts of reactive oxygen radicals after heat stress that attack the polyunsaturated fatty acids of the biomembrane, causing a chain reaction of lipid peroxidation, resulting in the amplification of free radicals and lipid peroxidation. The peroxidase lipid generated MDA by the decomposition of the peroxidase; MDA is widely used as an index of lipid peroxidation (Sehirli et al. 2008). MDA polymerization and crosslinking to form lipofuscin with proteins, peptides, or lipids caused changes in membrane structure and function and increased cell damage. The free radical NO synthesized in mammalian cells can cause oxidative injury in large numbers of cells. In addition, NOS can act on arginine to produce NO, which generates free radicals that are harmful to the body. Lipid peroxide can damage the plasma membrane, whereas normal cells do not release LDH from the cytoplasm, making LDH release a useful indicator of plasma membrane damage (Davila et al. 1998; Punyiczki and Fesus 1998; Jin 2007). Methionine inhibited the heat-induced LDH leakage.
Enzymatic radical scavenging systems are mainly involved in SOD, CAT, and GSH-Px. SOD is a family of antioxidant enzymes that act as the first line of cell defense against oxidative stress (Hu 2011), and SOD catalyzes the reaction of superoxide anion radicals generating H2O2 and O2. CAT further promotes the metabolism of H2O2 decomposing to the molecules O2 and H2O, thereby preventing attack by H2O2. GSH-Px catalyzes the peroxidation of GSH and clears the peroxide and hydroxyl radicals generated in the metabolic process of cellular respiration, thereby reducing the peroxidation of polyunsaturated fatty acids in the membrane, protecting the integrity of the membrane structure and function.
MDA, NO, NOS, LDH, SOD, CAT, and GSH-Px observation and analysis showed whether methionine had a protective effect on hyperthermia-treated BMECs and had a specific action on the mechanism combating oxidative stress. After adding different concentrations of methionine, the cells given 60 mg/L showed markedly reduced MDA, NO, NOS, and LDH formation and increased SOD, CAT, and GSH-Px enzymatic activity, which were all significantly different in the heat-treated cells not given methionine (p < 0.01). Methionine therefore effectively protects the integrity of the cell membrane. These results further support the antioxidant action of methionine in hyperthermia-induced toxicity in BMECs. The determination of the lipid peroxidation status in cultured BMECs after hyperthermia and methionine treatments indicated that the toxicity induced by heat stress through lipid peroxidation can be alleviated.
HSP70 is the highly inducible HSP belonging to the HSP70 family of cytoprotective proteins. Hsp70 participates in the regulation of apoptosis passing through the immune system, regulating enzymatic activity, and affecting related gene expression (Jaattela 1999). The increased expression of HSPs can protect against various kinds of cell damage, especially with regard to heat tolerance. Methionine is an extremely promising inducer of HSP, as it can increase HSP70 expression during heat stress, oxidative stress, and other stress states in cells or tissues and exhibits protective effects for cells or tissues under stress (Zhou 2010). This study found that after 24 h of culturing in 60 mg/L of methionine, the expression of HSP70 was significantly increased (p < 0.01) and inhibited the heat stress-induced apoptosis of BMECs, while increasing cell viability. The pretreatment with methionine may increase thermal tolerance through the induction of the expression of HSP70. Under normal circumstances, the synthesis and utilization of methionine in the body is via dynamic equilibrium, which prevents the loss of methionine. However, under severe stress, the methionine homeostasis is unbalanced, causing an excessive loss of methionine in damaged tissue and a rapid decline of immunity and disease resistance. Replenishing methionine can enhance the capability of resisting various diseases and stress. Thus, when the cell or organism succumbs to a nonfatal stress response, HSP70 overexpression may protect them from injury.
Apoptosis is programmed cell death controlled by gene expression. At present, the signal regulation of apoptosis is thought to have three key regulatory nodes: the Bcl-2 family, the caspase family, and mitochondria (Chipuk et al. 2010). The caspase family is the key component in the process of apoptosis; its activation and extraordinary expression induce apoptosis, and caspase-3 is situated downstream of the apoptotic cascade reaction, which is a convergence point transferring a variety of apoptosis signals (Gao and Suchida 1999; Yan et al. 2000). Bcl-2 inhibits the cytochrome C released by the mitochondria, hinders Apaf-1 binding with effector molecules, and blocks the apoptosis cascade (Li et al. 1997; Satoh et al. 2000). The benefits of methionine supplementation include an improvement in milk production and a reduction in the lymphocyte apoptosis, promotion of the expression of the Bcl-2 gene of the lymphocytes, and inhibition of the Bax gene (Han 2009). Rumen methionine supplementation in lactating cows can promote the proliferation of peripheral blood lymphocytes (Soder and Holden 1999).
This study found that pretreatment with methionine reduced the expression of Bax/Bcl-2 mRNA, prevented the increase of caspase-3 activity due to heat stress, and inhibited apoptosis induced by heat stress in BMECs. The Bcl-2 gene family includes important regulatory genes of apoptosis, while Bcl-2 has anti-apoptotic effects, and on the behalf of cell survival genes, Bax promotes apoptosis genes. These two proteins interact by forming a dimer network, where apoptosis is determined by the balance between different dimers; the higher the ratio of Bax/Bcl, the greater the likelihood of apoptosis. Amino acids can promote the synthesis and inhibit the breakdown of protein, which influences transcription to varying degrees. As a substrate of a protein, amino acid-regulated gene expression at the mRNA translation level plays an important regulatory role in mRNA translation at the initial stage through the plurality of signal channels and mechanisms.
The Bax-α gene is one of the genes that promotes apoptosis (Yonezawa et al. 2002), and at high temperatures, the abundance of Bcl-2 mRNA and Bax mRNA increased in the peripheral blood lymphocytes. Methionine can provide a methyl donor of DNA methylated in lymphocytes, where the 5′ end must form a cap structure in the process of mRNA, meaning that it is connected to a methylated guanosine by pyrophosphate in the mRNA terminal nucleotide. Furthermore, the riboses of the nucleotides connecting the cap structure also require different levels of methylation; the cap structure can protect the mRNA against the degradation of nucleotides and provide identification in the protein synthesis, thus increasing the abundance of mRNA (Nan et al. 1997). Adding methionine can increase Bax gene synthesis in the lymphocytes of the blood (Han 2009). Additionally, a lack of methionine can induce apoptosis in prostate cancer cells (Shan et al. 2002).
In conclusion, cultured BMECs provide a convenient and sensitive in vitro model for the assessment of hyperthermia-induced damage. Hyperthermia interferes with the integrity of cell membranes and causes cytotoxicity in cultured BMECs; however, methionine supplementation at 60 mg/L could block these deleterious effects through a reduction in MDA, NO, NOS, and LDH and an increase in SOD, CAT, and GSH-Px. Methionine supplementation increases the expression levels of HSP70 and HSF-1, reduces the expression of Bax/Bcl-2, thus increasing survival and decreasing apoptosis.
Acknowledgments
This study was supported by a grant from the National Supporting Projects for Science and Techniques (NO. 2012BAD12B10).
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Beere HM, Green DR. Stress management-heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 2001;11(1):6–10. doi: 10.1016/S0962-8924(00)01874-2. [DOI] [PubMed] [Google Scholar]
- Breillout F, Antoine E, Poupon MF. Methionine dependency of malignant tumors: a possible approach for therapy. J Nat Cancer Inst. 1990;82:1625–1632. doi: 10.1093/jnci/82.20.1628. [DOI] [PubMed] [Google Scholar]
- Chang MX. The impact of high temperatures on milk. China Herbivores. 2003;3:43–44. [Google Scholar]
- Chang SF, Sun YY, Yang LY. Bcl-2 gene family expression in the brain of rat offspring after gestational and lactational dioxin exposure. Ann N Y Acad Sci. 2005;1042:471–480. doi: 10.1196/annals.1338.040. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F. The Bcl-2 family reunion. Mol Cell. 2010;37(3):299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R, Collier J, Rhoads RP, Baumgard L. Genes involved in the bovine heat stress response. J Dairy Sci. 2008;91:445–454. doi: 10.3168/jds.2007-0540. [DOI] [PubMed] [Google Scholar]
- Crott J, Thomas P, Fenech M. Normal human lymphocytes exhibit wide rang of methionine-dependency which is related to altered cell division but not micronucleus frequency. J Mutagenesis. 2001;16:317–322. doi: 10.1093/mutage/16.4.317. [DOI] [PubMed] [Google Scholar]
- Davila JC, Rodriguez RJ, Melchert RB, Acosta DA. Predictive value of in vitro model systems in toxicology. Annu Rev Pharmacol. 1998;38:63–96. doi: 10.1146/annurev.pharmtox.38.1.63. [DOI] [PubMed] [Google Scholar]
- Du J, Di HS, Guo L. The effect of high temperature on mammary epithelial cells proliferation and apoptosis. Acta Zool Sin. 2006;52(5):959–965. [Google Scholar]
- Du J, Di HS, Wang GL. Establishment of a bovine epithelial mammary cell line and its ultrastructural changes when exposed to heat stress. Chin J Biotechnol. 2007;23:471–476. [PubMed] [Google Scholar]
- Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-H. [DOI] [PubMed] [Google Scholar]
- Gao CN, Suchida T. Activation of caspases in p53 induced transactivation-independent apoptosis. Jpn J Cancer Res. 1999;90(2):180–187. doi: 10.1111/j.1349-7006.1999.tb00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard CL, Lapierre H, Matte JJ, Lobley CE. Effect of dietary supplements of folic acid and rumen protected methionine on lactational performance and folate metabolism of dairy cows. J Dairy Sci. 2005;88:660–670. doi: 10.3168/jds.S0022-0302(05)72730-2. [DOI] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. Bcl-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13(15):1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Guo L. Effects of unitary or successive heat treatment on mammary epithelial cells proliferation and apoptosis. Journal of Nanjing Agricultural University. 2007;30:100–104. [Google Scholar]
- Halestrap AP, Mcstay GO, Clarke SJ. The permeability transition pore complex: another view. Biochimie. 2002;84(2):153–166. doi: 10.1016/S0300-9084(02)01375-5. [DOI] [PubMed] [Google Scholar]
- Han ZY. The effects of rumen-protected methionine on production performance of dairy cows, lymphocyte apoptosis and related gene under heat stress. Chinese Journal of Animal Nutrition. 2009;21:665–672. [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(12):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Hong YL. Cytoprotection of vitamin E on hyperthermia-induced damage in bovine mammary epithelial cells. Journal of Thermal. 2010;35:250–253. doi: 10.1016/j.jtherbio.2010.05.010. [DOI] [Google Scholar]
- Hu H. Responses of cultured bovine mammary epithelial cells to heat stress. J Agric Biotechnol. 2011;19:287–293. [Google Scholar]
- Jaattela M. Heat shock proteins as cellular lifeguards. Ann Med. 1999;31:261–271. doi: 10.3109/07853899908995889. [DOI] [PubMed] [Google Scholar]
- Jin HM. Pathology and physiology. National Med J China. 2007;76:945–946. [Google Scholar]
- Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: a short introduction to newcomers in the field. Int J Hyperthermia. 2006;22(3):191–196. doi: 10.1080/02656730500532028. [DOI] [PubMed] [Google Scholar]
- Koremer G, Zamzami N, Susin SA. Mitochondria control of apoptosis. Immunol Today. 1997;18:44–51. doi: 10.1016/S0167-5699(97)80014-X. [DOI] [PubMed] [Google Scholar]
- Lepock JR. How do cells respond to their thermal environment? Int J Hyperthermia. 2005;21(8):681–687. doi: 10.1080/02656730500307298. [DOI] [PubMed] [Google Scholar]
- Li XY. The effect of lysine and methionine on proliferation of bovine mammary epithelial cells in vitro by MTT colorimetric assay. Biotechnol Bull. 2010;3:143–148. [Google Scholar]
- Li P, Nijhawan D, Budiardjo I. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–489. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Lin H, Cao ZJ, Wang L. Responses of milk protein and mammary amino acids metabolism to duodenal soybean small peptides and free amino acids infusion in lactating goat. J Dairy Sci. 2009;92(E-suppl 1):47. [Google Scholar]
- Lindquist S, Craig EA. The heat shock protein. Ann Rev Genet. 1988;22:631–637. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Liu GS. Temperature effects on milk yield and measure. Anim Sci and Vet Med. 2004;6:26–28. [Google Scholar]
- Liu HY, Yang JY, Wu HH. Effects of methionine and its ratio to lysine on expression of αs1-casein gene in cultured bovine mammary epithelial cells. J Anim Feed Sci. 2007;16:330–334. [Google Scholar]
- Lobley CG, Connell A, Buchan V. Administration of testosterone to wether lambs: effect on protein and energy metabolism and growth hormone status. J Endocrinol. 1987;115:439. doi: 10.1677/joe.0.1150439. [DOI] [PubMed] [Google Scholar]
- Mercier JC, Vilott JL. Structure and function of milk protein genes. J Dairy Sci. 1993;76:3079–3098. doi: 10.3168/jds.S0022-0302(93)77647-X. [DOI] [PubMed] [Google Scholar]
- Morano KA, Thiele DJ. Heat Shock Factor function and regulation in response to cellular stress, growth, and differentiation signals. Gene Exp. 1999;7:271–282. [PMC free article] [PubMed] [Google Scholar]
- Mu T, Kong GH, Han ZY, Li HX. Cytoprotection of methionine on hyperthermia-induced damage in bovine mammary epithelial cells. Cell Biol Int. 2014;38:971–976. doi: 10.1002/cbin.10271. [DOI] [PubMed] [Google Scholar]
- Nan X, Campoy FJ, Bird A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell. 1997;88:471–481. doi: 10.1016/S0092-8674(00)81887-5. [DOI] [PubMed] [Google Scholar]
- Nichols JR, Schingoephe DJ, Maiga HA, Brouk MJ, Piepenbrink MS. Evaluation of corn distillers grains and ruminally protected lysine and methionine for lactating dairy cows. J Dairy Sci. 1998;81:482–491. doi: 10.3168/jds.S0022-0302(98)75600-0. [DOI] [PubMed] [Google Scholar]
- Nutt LK, Pataer A, Pahler J. Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. J Biol Chem. 2002;277(11):9219–9225. doi: 10.1074/jbc.M106817200. [DOI] [PubMed] [Google Scholar]
- Patel MP, Masood A, Patel PS. Targeting the Bcl-2. Curr Opin Oncol. 2009;21(6):516–523. doi: 10.1097/CCO.0b013e328331a7a4. [DOI] [PubMed] [Google Scholar]
- Punyiczki M, Fesus L. The two defense systems of the organism may have overlapping molecular elements. Ann N Y Acad Sci. 1998;851:67–74. doi: 10.1111/j.1749-6632.1998.tb08978.x. [DOI] [PubMed] [Google Scholar]
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Satoh K, Kaneko K, Hirota M. The pattern of CPP32/caspase-3 expression reflects the biological behavior of the human pancreatic duct cell tumors. Pancreas. 2000;21(4):352–357. doi: 10.1097/00006676-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Sehirli O, Tozan A, Omurtag GZ. Protective effect of resveratrol against naphthalene-induced oxidative stress in mice. Ecotoxicol Environ Saf. 2008;71:301. doi: 10.1016/j.ecoenv.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Shan L, Sara MH, Eugene MC, Danial EE. Methionine restriction induces apoptosis of prostate cancer cells via the c-Jun N-terminal kinase mediated signaling pathway. Cancer Letter. 2002;179:51–58. doi: 10.1016/S0304-3835(01)00852-7. [DOI] [PubMed] [Google Scholar]
- Soder KJ, Holden LA. Lymphocyte proliferation response of lactating dairy cows fed varying concentrations of rumen protection methionine. J Dairy Sci. 1999;82:1935–1942. doi: 10.3168/jds.S0022-0302(99)75429-9. [DOI] [PubMed] [Google Scholar]
- Sonn L, Fujita J, Gaffin SL. Invited review: effects of heat and cold stress on mammalian gene expression. J Appl Physiol. 2002;92:1725–1742. doi: 10.1152/japplphysiol.01143.2001. [DOI] [PubMed] [Google Scholar]
- Sun PM, Wang ZL, Li JM. The new development of heat shock protein 70. Chin J Vet Sci. 2007;27(2):284–288. [Google Scholar]
- Wang LX. Regulation and detecting methods of cell apoptosis. J Anim Sci and Vet Med. 2007;26:40–42. [Google Scholar]
- Wang JP. Effects of heat stress on cows progress. Chin Dairy. 2008;7:21–24. [Google Scholar]
- Wang JL. Effect of methionine on lactation ability of dairy cow mammary epithelial cells cultured in vitro. J Dairy Sci and Technol. 2012;35:8–10. [Google Scholar]
- Wang C, Liu JX (2008) Effects of ratio of lysine to methionine in metabolizable protein on lactation performance of dairy COWS. Proceeding of the 13th Animal Science Congress of the Asian-Australian of Animal Production Societies 65
- Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Bio. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- Wu HH, Zhao XJ, Zhang CQ. Establishment of mammary tissue culture: an in vitro mode for bovine lactation. J Anim Feed Sci. 2004;13:575–578. [Google Scholar]
- Yan XY, Li XH. Study on the heat stress in dairy cows. Anim Sci & Vet Med. 2004;9(21):42–44. [Google Scholar]
- Yan Z, Cynthia G, Andren LB. Selective and protracted apoptosis in human primary neurons microinjection with active caspase-3, -6, -7 and -8. J Neurosci. 2000;20:8384–8389. doi: 10.1523/JNEUROSCI.20-22-08384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JY. Effects of methionine and methionylmethionine on expression of casein αs1 gene in cultured bovine mammary epithelial cells. J Agr Biotechnol. 2007;1:24–27. [Google Scholar]
- Yonezawa M, Otsuka T, Kato T, Moriyama A, Kato KH, Asai K, Matsui N. Hyperthermic induction of apoptosis in malignant fibrous histiocytoma cells: possible involvement of a p53-independent pathway in induction of Bax gene. Ortthop Sci. 2002;7(1):117–122. doi: 10.1007/s776-002-8432-4. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Lv ZH, Li JL, Li S, Xu SW, Wang XL. Effects of cold stress on nitric oxide in duodenum of chicks. Poult Sci. 2011;90(7):1555–1561. doi: 10.3382/ps.2010-01333. [DOI] [PubMed] [Google Scholar]
- Zhou ZF. Cell growth, apoptosis and the mRNA transcription of heat shock protein: effects of heat stress on bovine mammary epithelial cells. Acta Veterinariaet Zootechnica Sinica. 2010;41:600–607. [Google Scholar]