Figure 5.
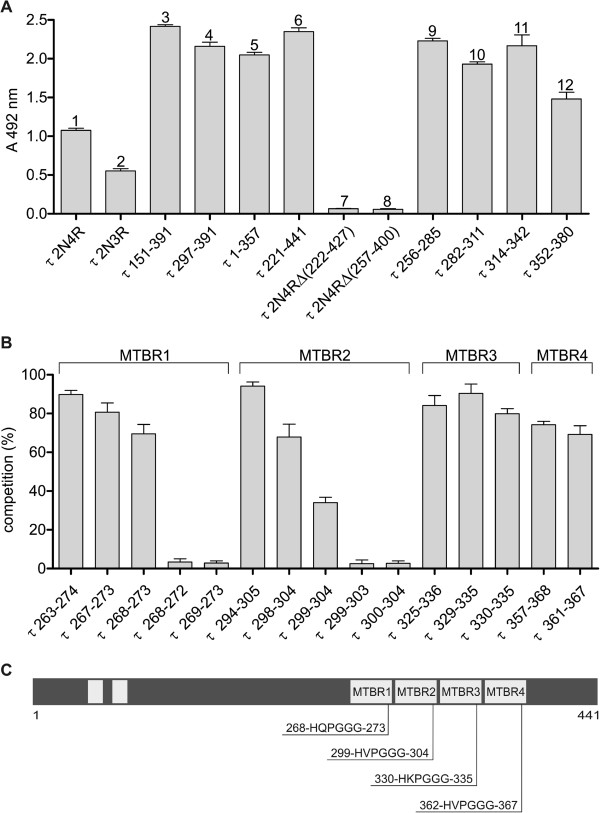
Mapping of domain on tau protein recognised by DC8E8. (A) For the epitope mapping of DC8E8 monoclonal antibody, we used full-length three- and four-repeat tau isoforms with two N-terminal inserts (1 and 2), tau deletion mutants (3–8) and tau-derived synthetic peptides (9–12). All deletion mutants that contained the microtubule-binding repeat (MTBR) region (3–6) were recognized by DC8E8; tau deletion mutants lacking the MTBR region were not recognized (8 and 9). Importantly, DC8E8 recognized each of synthetic peptides derived from individual MTBRs (9–12). The result is that DC8E8 recognised four binding sites (epitopes) located in the MTBR region of the tau protein, and each epitope is separately located within one MTBR. (B) To narrow down the DC8E8 minimal epitope, homologous peptides derived from the tau protein repeat region (MTBR1–4) were analysed in competitive enzyme-linked immunosorbent assays. Tau peptides containing at least six amino acids of the DC8E8 recognition sequence HXPGGG were capable of competing with mis-disordered tau (amino acids 151–391) for binding to antibody DC8E8. Tau peptides containing five amino acids of the DC8E8 recognition sequence did not compete with mis-disordered tau for binding to antibody DC8E8. (C) Schema of epitopes on tau protein molecule recognised by DC8E8. The DC8E8 monoclonal antibody is capable of binding four separate binding regions, with each region forming one individual epitope. These four epitopes are separately located within the first, second, third and fourth MTBR of protein tau. Note: All listed molecules are numbered in reference to the longest human tau isoform (2N4R) sequence.
