Figure 6.
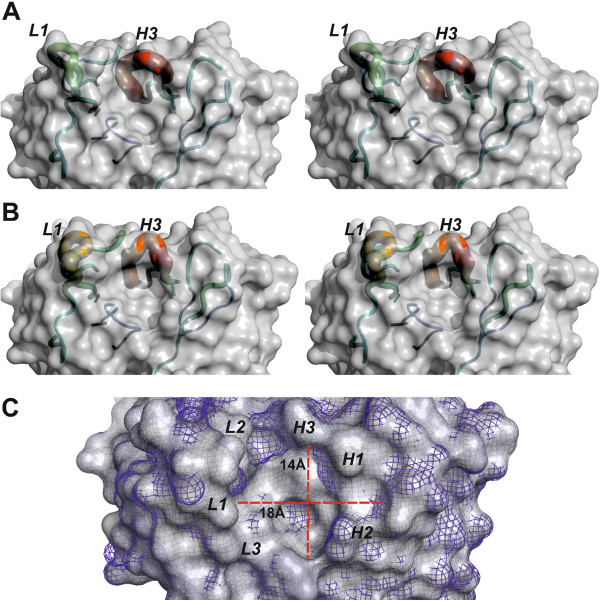
The flexibility of the binding site allows DC8E8 to adapt to four homologous, albeit not identical, structural determinants in the tau microtubule-binding repeats. Two independently refined X-ray structures of DC8E8 antigen-binding fragment (A) and (B) (stereoview) show the flexibility of the antigen-binding site. The surface of the antibody is shown in grey. The backbones of complementarity determining regions (CDRs) are represented as tubes, with the diameter and colour reflecting their averaged atomic displacement parameters, that is, flexibility. The flexibility is expressed as a colour scale ranging from blue to red, corresponding to B-factors 30 to 150 Å2. The CDRs L1 and H3 exhibit higher B-factors than the remaining parts of the model. The pronounced flexibility of these CDRs is essential to allowing DC8E8 to bind each of four slightly different epitopes within the microtubule-binding repeats (MTBRs). (C) Superposition of both independently refined DC8E8 molecules shows that the core of the binding pockets is invariant (molecule A shown as grey solid, molecule B as blue mesh). The CDR loops (italic letters) create a 7- to 9-Å-deep pocket with surface dimensions 18 × 14 Å (red axes). The shape of this pocket necessitates that the minimal DC8E8 epitope HXPGGG adopts a fold protruding into this space to bind in the DC8E8 combining site.
