Figure 7.
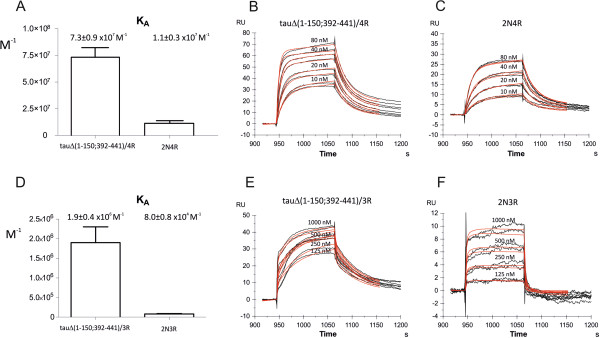
DC8E8 discriminates between pathological and physiological ‘healthy’ tau. (A) Affinity comparison of DC8E8–four-repeat tau protein complex formation. The monoclonal antibody DC8E8 exhibits preferential affinity to the mis-disordered truncated tau 151-391/4R. Surface plasmon resonance (SPR) revealed that mis-disordered truncated tau protein is recognised by DC8E8 nearly seven times stronger than the full-length tau protein isoform. Extrapolation of kinetic SPR sensorgrams of DC8E8 interaction with mis-disordered truncated (B) and full-length (C) four-repeat tau proteins revealed that mis-disordered truncated tau is recognised by DC8E8 with kon = 2.9 × 106 M−1 s−1 and koff = 0.04 s−1, whereas full-length tau exhibits kon = 4.4 × 105 M−1 s−1 and koff = 0.04 s−1. (D) DC8E8 affinity comparison of three-repeat truncated tau and its full-length counterpart. Similarly to the four-repeat tau, extrapolation of kinetic SPR sensorgrams of DC8E8 interaction with truncated (E) and full-length (F) three-repeat tau proteins revealed that truncated tau is recognised by DC8E8 with kon = 1.5 × 105 M−1 s−1 and koff = 0.08 s−1, whereas full-length tau exhibits kon = 2.8 × 104 M−1 s−1 and koff = 0.4 s−1. These results show that three-repeat mis-disordered truncated tau protein is recognised by DC8E8 with 25 times higher affinity than full-length three-repeat tau protein. Black curves represent experimental data, and red curves were fitted by evaluation software for kinetic parameter calculations.
