Abstract
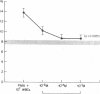
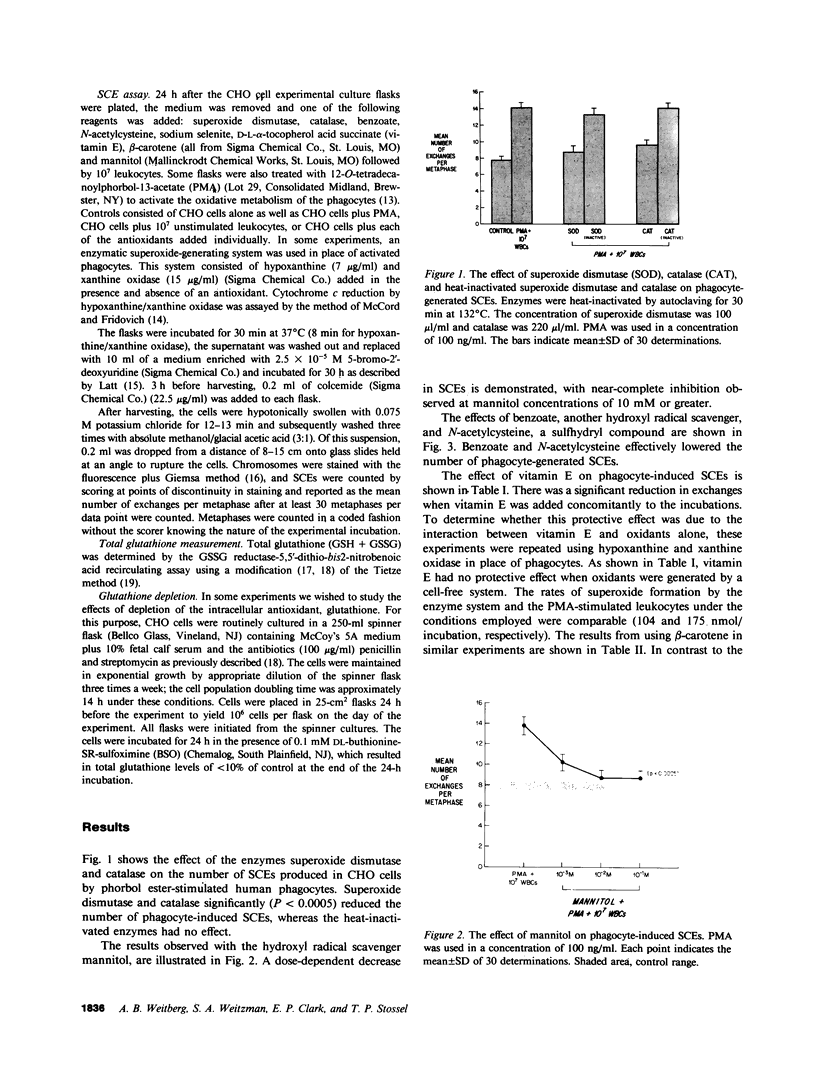
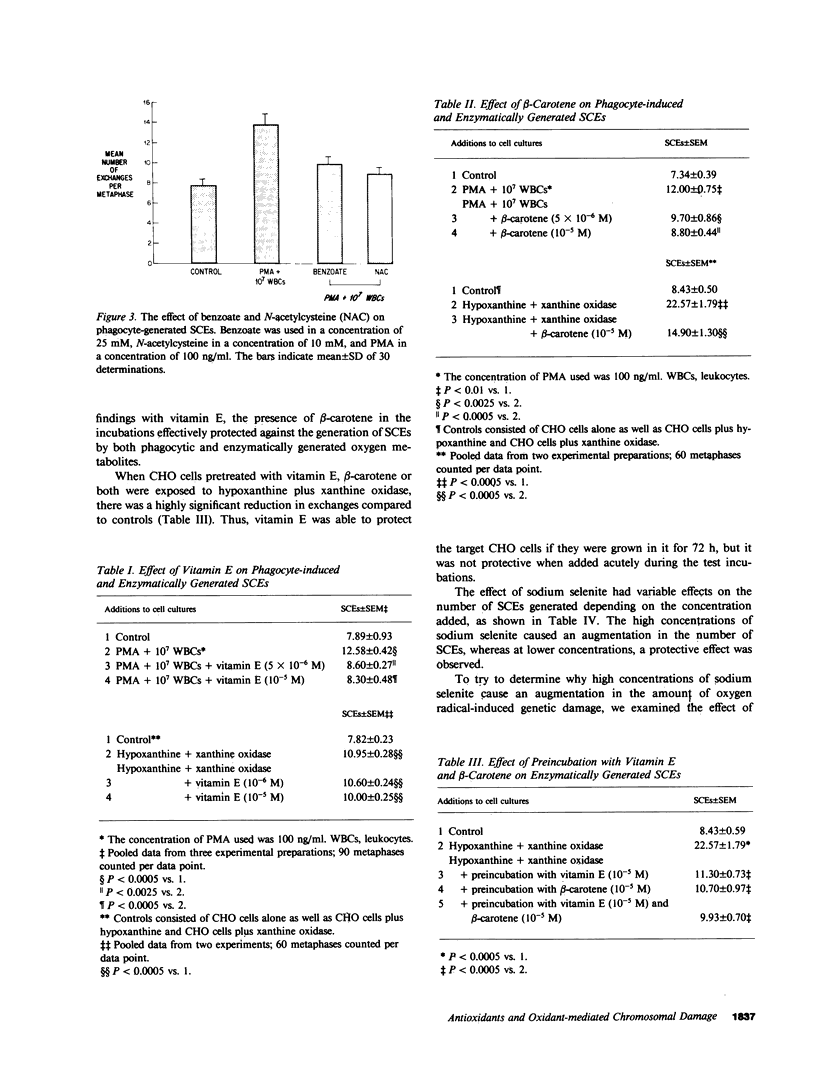
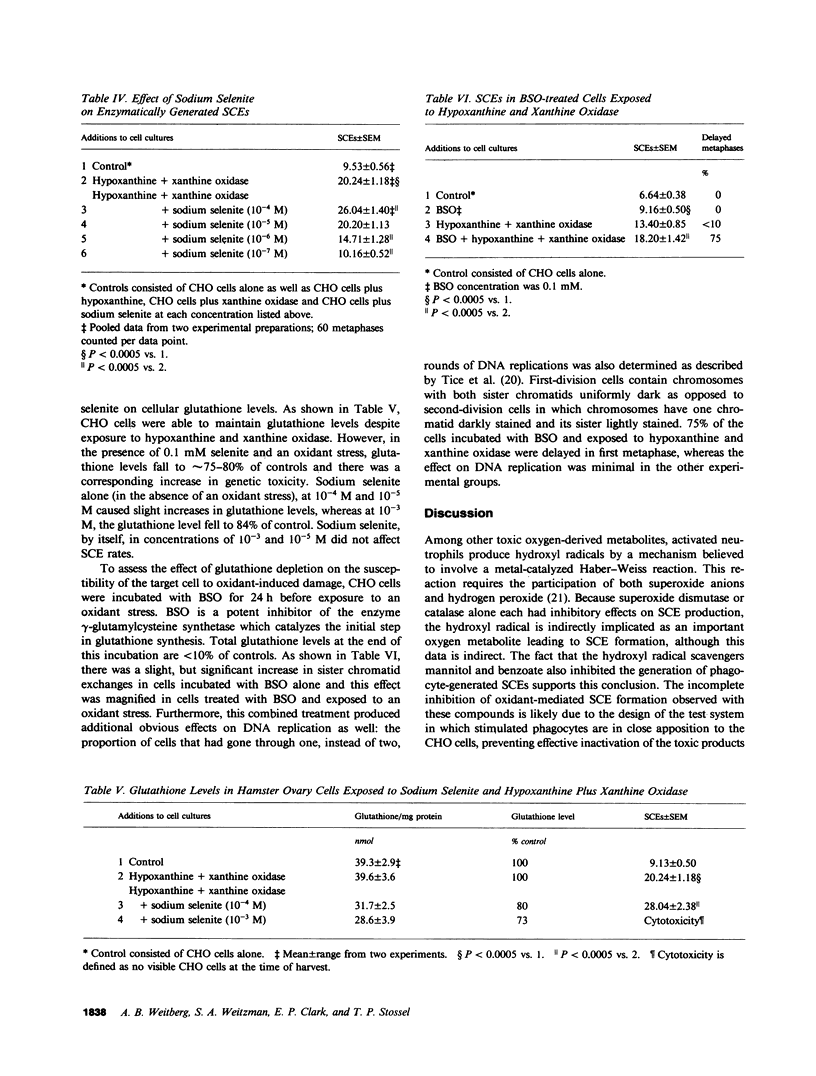
Stimulated human phagocytes produce sister chromatid exchanges in cultured mammalian cells by a mechanism involving oxygen metabolites. Experiments were designed to determine whether antioxidants inhibit this process. Superoxide dismutase, catalase, and hydroxyl radical scavengers (benzoate, mannitol) protected target Chinese hamster ovary cells from phagocyte-induced sister chromatid exchanges, implicating the involvement of hydroxyl radicals in this chromosomal damage. N-acetylcysteine and beta-carotene were also protective. alpha-Tocopherol (greater than 5 microM) protected target cells exposed to phagocytes but not to enzymatically generated oxidants when the vitamin was added just before the source of oxygen radicals, suggesting, as reported by others, that the principal action of tocopherol in this setting was to inhibit the release of oxidants from phagocytes. On the other hand, cultivation of target cells with supplemental tocopherol protected them from the toxic effects of the enzymatic oxidant-producing system, indicating a role for membrane-associated free radicals in the mechanism of sister chromatid exchange induction. Low concentrations of sodium selenite (0.1-1.0 microM) protected the target cells. However, higher concentrations (10 microM) of selenite had no effect on oxidant-induced sister chromatid exchange formation, and 0.1 mM selenite increased the number of exchanges. Sodium selenite concentrations of 0.1 mM also decreased the intracellular glutathione concentration of target cells during an oxidant stress, and reducing target cell glutathione concentrations with buthionine sulfoximine increased their sensitivity to oxygen-related chromosomal damage. Therefore, the potentiation of oxygen radical-induced chromosomal damage observed with high concentrations of selenite may result from a decrease in the thiol antioxidant defense systems within the cell. The findings suggest that the hydroxyl radical has an important role in the production of phagocyte-induced cytogenetic injury, membrane-derived intermediates may be involved, depletion of intracellular glutathione renders cells more susceptible to this injury, and supplementation of target cells with antioxidants can protect them from oxygen radical-generated chromosomal injury.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrick B. A., Nathan C. F., Griffith O. W., Cohn Z. A. Glutathione depletion sensitizes tumor cells to oxidative cytolysis. J Biol Chem. 1982 Feb 10;257(3):1231–1237. [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Bieri J. G., Corash L., Hubbard V. S. Medical uses of vitamin E. N Engl J Med. 1983 May 5;308(18):1063–1071. doi: 10.1056/NEJM198305053081805. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Ingold K. U. beta-Carotene: an unusual type of lipid antioxidant. Science. 1984 May 11;224(4649):569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- Butterick C. J., Baehner R. L., Boxer L. A., Jersild R. A., Jr Vitamin E--a selective inhibitor of the NADPH oxidoreductase enzyme system in human granulocytes. Am J Pathol. 1983 Sep;112(3):287–293. [PMC free article] [PubMed] [Google Scholar]
- Chow C. K. Nutritional influence on cellular antioxidant defense systems. Am J Clin Nutr. 1979 May;32(5):1066–1081. doi: 10.1093/ajcn/32.5.1066. [DOI] [PubMed] [Google Scholar]
- Clark E. P., Epp E. R., Biaglow J. E., Morse-Gaudio M., Zachgo E. Glutathione depletion, radiosensitization, and misonidazole potentiation in hypoxic Chinese hamster ovary cells by buthionine sulfoximine. Radiat Res. 1984 May;98(2):370–380. [PubMed] [Google Scholar]
- Fridovich S. E., Porter N. A. Oxidation of arachidonic acid in micelles by superoxide and hydrogen peroxide. J Biol Chem. 1981 Jan 10;256(1):260–265. [PubMed] [Google Scholar]
- Goldstein B. D., Witz G., Amoruso M., Stone D. S., Troll W. Stimulation of human polymorphonuclear leukocyte superoxide anion radical production by tumor promoters. Cancer Lett. 1981 Jan;11(3):257–262. doi: 10.1016/0304-3835(81)90117-8. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip C. Prophylaxis of mammary neoplasia by selenium supplementation in the initiation and promotion phases of chemical carcinogenesis. Cancer Res. 1981 Nov;41(11 Pt 1):4386–4390. [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- Klock J. C., Stossel T. P. Detection, pathogenesis, and prevention of damage to human granulocytes caused by interaction with nylon wool fiber. Implications for filtration leukapheresis. J Clin Invest. 1977 Nov;60(5):1183–1190. doi: 10.1172/JCI108871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S. A. Sister chromatid exchanges, indices of human chromosome damage and repair: detection by fluorescence and induction by mitomycin C. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3162–3166. doi: 10.1073/pnas.71.8.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo L. W., Koropatnick J., Stich H. F. The mutagenicity and cytotoxicity of selenite, "activated" selenite and selenate for normal and DNA repair-deficient human fibroblasts. Mutat Res. 1978 Mar;49(3):305–312. doi: 10.1016/0027-5107(78)90103-3. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCullough J., Weiblen B. J., Deinard A. R., Boen J., Fortuny I. E., Quie P. G. In vitro function and post-transfusion survival of granulocytes collected by continuous-flow centrifugation and by filtration leukapheresis. Blood. 1976 Aug;48(2):315–326. [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Mitchell J. B., Russo A., Kinsella T. J., Glatstein E. Glutathione elevation during thermotolerance induction and thermosensitization by glutathione depletion. Cancer Res. 1983 Mar;43(3):987–991. [PubMed] [Google Scholar]
- Packer J. E., Mahood J. S., Willson R. L., Wolfenden B. S. Reactions of the trichloromethylperoxy free radical (Cl3COO) with tryptophan, tryptophanyl-tyrosine and lysozyme. Int J Radiat Biol Relat Stud Phys Chem Med. 1981 Feb;39(2):135–141. doi: 10.1080/09553008114550151. [DOI] [PubMed] [Google Scholar]
- Perry P., Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974 Sep 13;251(5471):156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Pietronigro D. D., Barrie W., Jones G., Kalty K., Demopoulos H. B. Interaction of DNA and liposomes as a model for membrane-mediated DNA damage. Nature. 1977 May 5;267(5606):78–79. doi: 10.1038/267078a0. [DOI] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J. H., Altenburg L. C. Sister-chromatid exchange induction by sodium selenite: dependence on the presence of red blood cells or red blood cell lysate. Mutat Res. 1978 Dec;54(3):343–354. doi: 10.1016/0165-1161(78)90024-9. [DOI] [PubMed] [Google Scholar]
- Ray J. H., Altenburg L. C. Sister-chromatid exchange induction by sodium selenite: plasma protein-bound selenium is not the active SCE-inducing metabolite of Na2SeO3. Mutat Res. 1982 Oct-Nov;102(3):285–296. doi: 10.1016/0165-1218(82)90138-0. [DOI] [PubMed] [Google Scholar]
- Rinehart J. J., Lange P., Gormus B. J., Kaplan M. E. Human monocyte-induced tumor cell cytotoxicity. Blood. 1978 Jul;52(1):211–220. [PubMed] [Google Scholar]
- Rosin M. P., Stich H. F. Assessment of the use of the Salmonella mutagenesis assay to determine the influence of antioxidants on carcinogen-induced mutagenesis. Int J Cancer. 1979 May 15;23(5):722–727. doi: 10.1002/ijc.2910230521. [DOI] [PubMed] [Google Scholar]
- Shamberger R. J., Baughman F. F., Kalchert S. L., Willis C. S., Hoffman G. C. Carcinogen-induced chromosomal breakage decreased by antioxidants. Proc Natl Acad Sci U S A. 1973 May;70(5):1461–1463. doi: 10.1073/pnas.70.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamberger R. J., Corlett C. L., Beaman K. D., Kasten B. L. Antioxidants reduce the mutagenic effect of malonaldehyde and beta-propiolactone. Part IX. Antioxidants and cancer. Mutat Res. 1979 Apr;66(4):349–355. doi: 10.1016/0165-1218(79)90045-4. [DOI] [PubMed] [Google Scholar]
- Shamberger R. J. Relationship of selenium to cancer. I. Inhibitory effect of selenium on carcinogenesis. J Natl Cancer Inst. 1970 Apr;44(4):931–936. [PubMed] [Google Scholar]
- Shamberger R. J., Tytko S. A., Willis C. E. Antioxidants and cancer. Part VI. Selenium and age-adjusted human cancer mortality. Arch Environ Health. 1976 Sep-Oct;31(5):231–235. doi: 10.1080/00039896.1976.10667225. [DOI] [PubMed] [Google Scholar]
- Speit G., Vogel W. The effect of sulfhydryl compounds on sister-chromatid exchanges. II. The question of cell specificity and the role of H2O2. Mutat Res. 1982 Mar;93(1):175–183. doi: 10.1016/0027-5107(82)90133-6. [DOI] [PubMed] [Google Scholar]
- Tice R., Schneider E. L., Rary J. M. The utilization of bromodeoxyuridine incorporation into DNA for the analysis of cellular kinetics. Exp Cell Res. 1976 Oct 15;102(2):232–236. doi: 10.1016/0014-4827(76)90037-9. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Weitberg A. B., Weitzman S. A., Destrempes M., Latt S. A., Stossel T. P. Stimulated human phagocytes produce cytogenetic changes in cultured mammalian cells. N Engl J Med. 1983 Jan 6;308(1):26–30. doi: 10.1056/NEJM198301063080107. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Stossel T. P. Effects of oxygen radical scavengers and antioxidants on phagocyte-induced mutagenesis. J Immunol. 1982 Jun;128(6):2770–2772. [PubMed] [Google Scholar]
- Weitzman S. A., Stossel T. P. Mutation caused by human phagocytes. Science. 1981 May 1;212(4494):546–547. doi: 10.1126/science.6259738. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Weitberg A. B., Clark E. P., Stossel T. P. Phagocytes as carcinogens: malignant transformation produced by human neutrophils. Science. 1985 Mar 8;227(4691):1231–1233. doi: 10.1126/science.3975611. [DOI] [PubMed] [Google Scholar]
- Whiting R. F., Wei L., Stich H. F. Unscheduled DNA synthesis and chromosome aberrations induced by inorganic and organic selenium compounds in the presence of glutathione. Mutat Res. 1980 Jun;78(2):159–169. doi: 10.1016/0165-1218(80)90095-6. [DOI] [PubMed] [Google Scholar]
- Zimmerman R., Cerutti P. Active oxygen acts as a promoter of transformation in mouse embryo C3H/10T1/2/C18 fibroblasts. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2085–2087. doi: 10.1073/pnas.81.7.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]