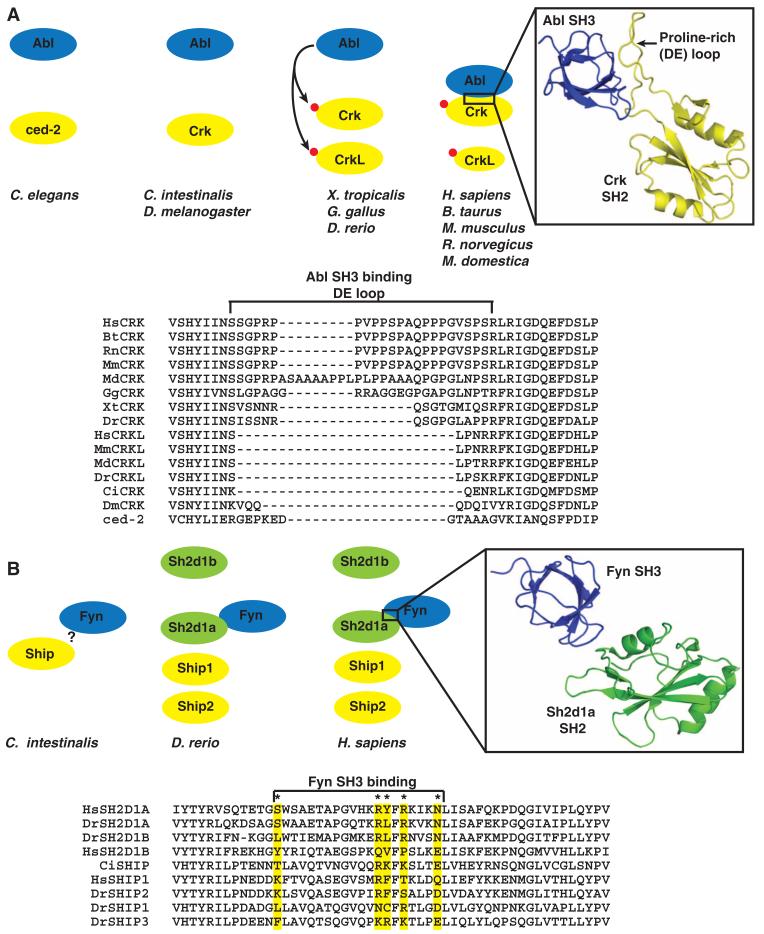
Fig. 5.
Evolving new SH2 interactions between duplicate genes. (A) In vertebrates, both Crk and CrkL contain a tyrosine residue that is phosphorylated by Abl or other tyrosine kinases (red circle). Phosphorylation on this conserved site promotes the intramolecular interaction with the respective SH2 domain. In mammals, but not teleosts, the Crk SH2 domain has evolved a loop between β strands D and E (DE loop) that creates a binding site for the SH3 domain of Abl to promote this interaction and phosphorylation of Crk. A ribbon diagram shows the human Crk (yellow) SH2 domain with the extended proline-rich loop interacting with the SH3 domain of Abl (blue). The structure was adapted from PDB 1JU5. The sequences of the SH2 domain from the CRK family in C. elegans (ced-2), D. melanogaster (DmCrk), C. intestinalis (CiCrk), D. rerio (DrCrk, DrCrkL), M. domestica (MdCrk, MdCrkL), Bos taurus (BtCrk, BtCrkL), M. musculus, (MmCrk, MmCrkL), and H. sapiens (HsCrk, HsCrkL) were aligned with ClustalX. For a detailed sequence analysis, see also fig. S6B. (B) The Sh2d1a and Sh2d1b adaptors arose through gene duplication and domain loss from the Ship-encoding gene in C. intestinalis, resulting in four related SH2 domains in vertebrates. The SH3 domain of Fyn makes contact with residues within the SH2 domain of Sh2d1a, but not with Sh2d1b and Ship. These contact residues are conserved in only vertebrate Sh2d1a. The structure was adapted from PDB1M27. The * indicates key contact residues previously reported (48).