Figure 4.
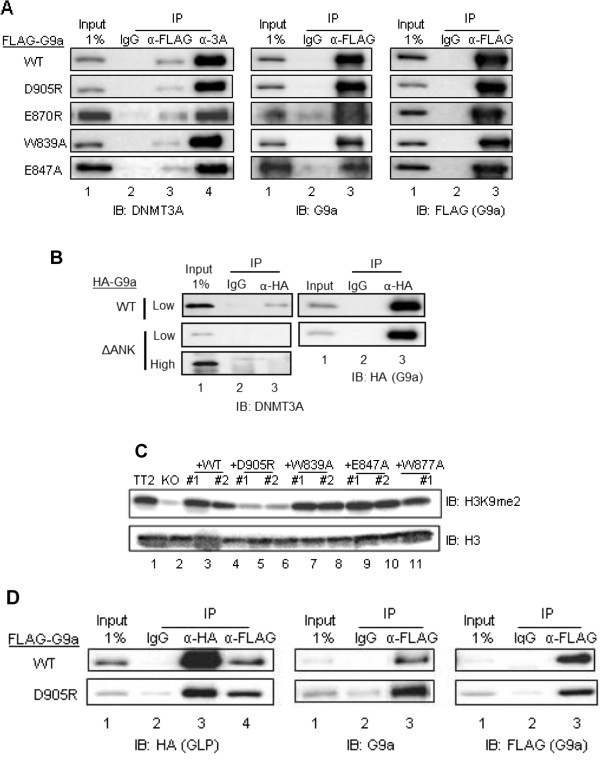
Mutations in the histone H3 binding surface of the G9a ankyrin repeat (ANK) domain do not abrogate binding to DNMT3A. COS- 7 cells were transiently transfected with pcDNA3.1-mDNMT3A (A and B) or pSG5-HA-mGLP full-length (D) and pSG5-FLAG-mG9a full-length (A, B and D) or pSG5-HA-mG9a.ΔANK (B). G9a was either wild-type (WT) or contained the indicated point mutations in the ANK domain. G9a, GLP and DNMT3A were immunoprecipitated from cell extracts with an anti-FLAG/anti-HA, anti-HA or anti-DNMT3A antibody, respectively. Non-immune IgG antibody was used for immunoprecipitation background estimation. Bound proteins were analyzed by immunoblot (IB) with the indicated antibodies. A 1% input sample was loaded for comparison. High (3 minutes) and low (1 minute) exposure times are shown (B). (C) Immunoblots were used to determine H3K9me2 and H3 protein levels in acid histone extracts from the indicated undifferentiated mESC lines.
