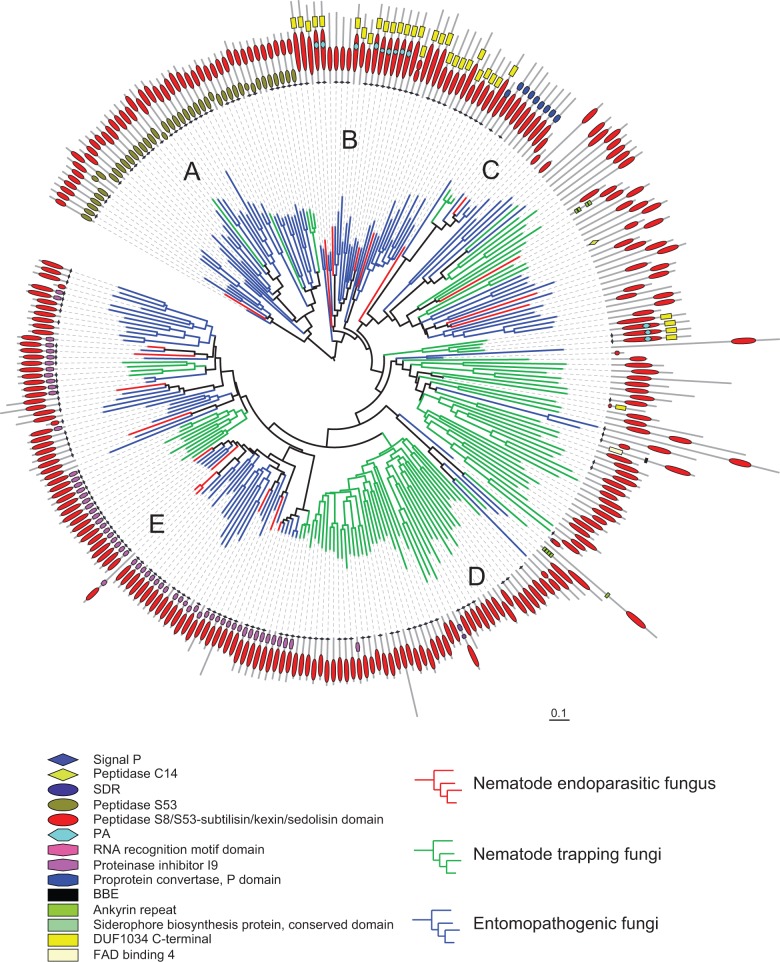
Fig. 3.—
Phylogenetic tree of nematophagous and insect fungal proteinases containing peptidase S8/S53-subtilisin/kexin/sedolisin domains annotated by Pfam. Proteinase genes from one nematode endoparasitic fungus, three nematode-trapping fungi, and five insect fungi are labeled in branch with red, green, and blue color, respectively. Phylogeny was estimated using RAxML (see Materials and Methods). Five clades were identified according to the different domain architecture of proteinase genes. Genes in Clade A contain peptidase S53 domains in combination with the peptidase S8/S53-subtilisin/kexin/sedolisin domains. This combination is found mainly in insect fungi. Most of proteinase genes in nematode endoparasitic fungus are located in Clades B–E. They have more close relationship with those of insect fungi than with trapping fungi. Clades B and C have longer peptidase S8/S53-subtilisin/kexin/sedolisin domains compared with other clades and contain DUF1034 C-terminal and proprotein convertase P domains closest to the conserved domain, respectively. Specially nine genes in Clade B contain PA signature (protease-associated domain) embedded within the peptidase S8/S53 domain. Clade D may have arisen differently; as the majority of proteins belong to nematode-trapping fungi do not have any other domains in combination with the peptidase S8/S53 domain. Half of the genes in Clade D do not carry a signal peptide, thus they do not seem to function extracellularly. Clade E is an expanded group of genes that contain signal peptide and proteinase inhibitor I9 domain on the N-terminal of proteins.