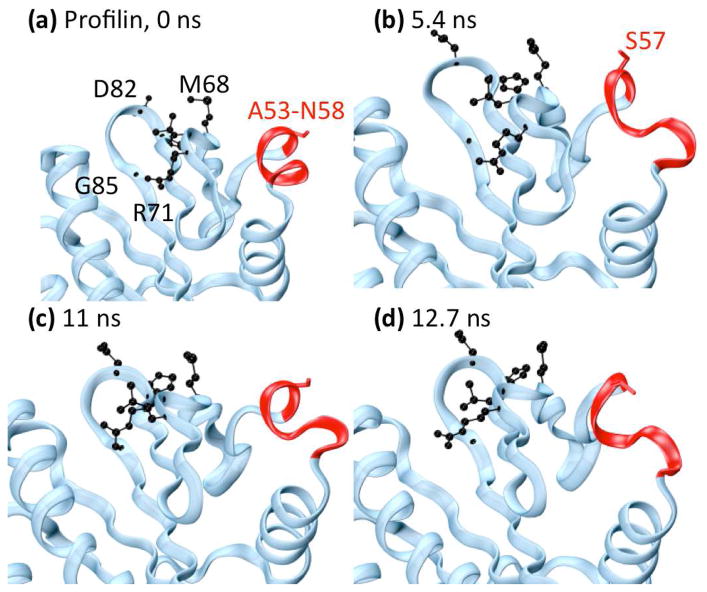
Fig. 6.
Changes in profilin (ProF) binding residue S57. Helix α3, containing S57, is shown in red. Side-chains of actin binding residues highlighted by wavelet analysis are shown in black, and the side-chain of S57 is shown in red. (a) Minimized crystal structure. (b) 5.4 ns, (c) 11 ns, and (d) 12.7 ns. During this time period, helix α3 twists significantly and unravels from the N-terminal end, changing the orientation of S57 to the binding site.