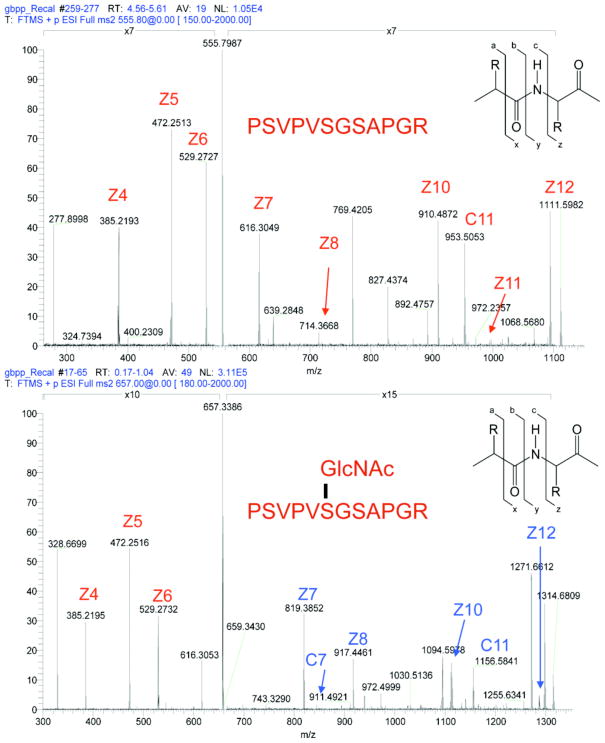
Fig. 2. Site-mapping of O-GlcNAc sites is facilitated by electron dissociation techniques.
UL32, a synthetic O-GlcNAc modified protein, is efficiently fragmented and the site of modification (from three possible sites) is easily assigned via electron capture dissociation. When comparing the spectra from unglycosylated (top) and glycosylated peptide (bottom), singly charged fragments retaining the O-GlcNAc modified serine (shown in BLUE) show an increase in mass to charge of 203 daltons, the weight of a single GlcNAc residue.