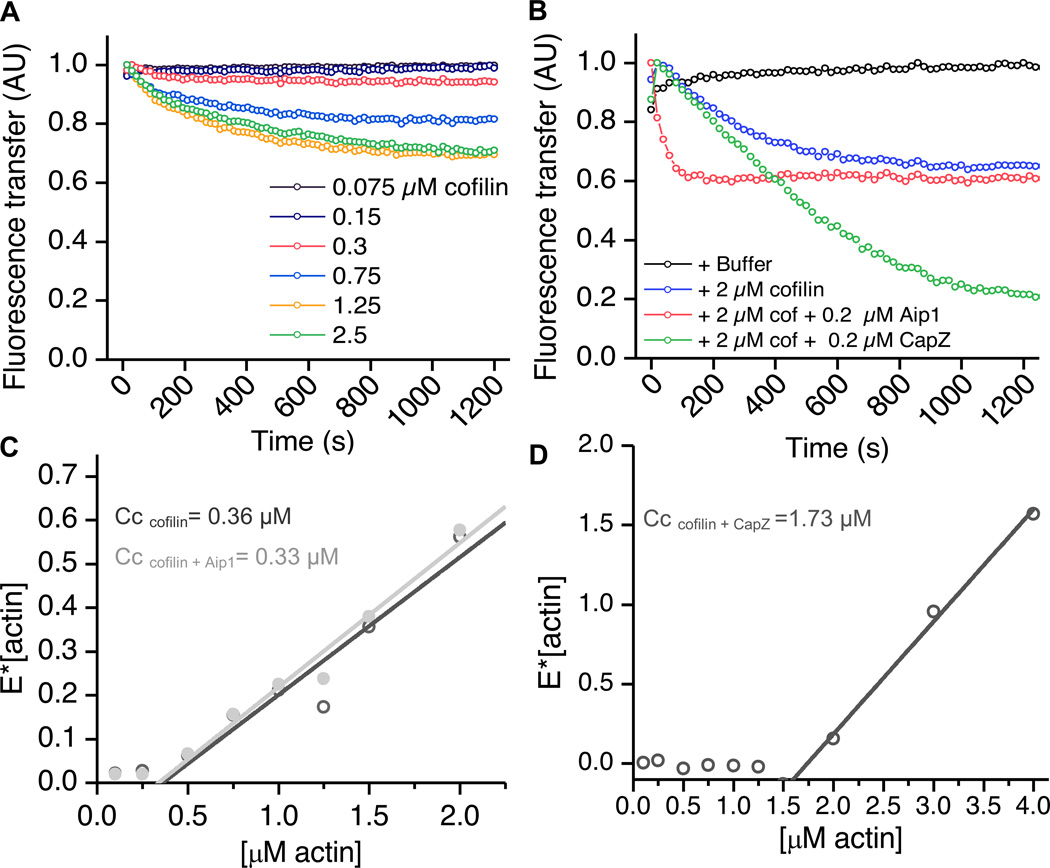
FIGURE 5.
CapZ and Aip1 have differing effects on the rates and extents of cofilin-mediated disassembly. (A) Increasing concentrations of cofilin cause an initial disassembly of actin in a dose-dependent manner. 2 µM pre-polymerized F-actin was mixed with varying amounts of cofilin, and the fluorescence of Oregon green actin was measured over time. The decrease in FRET represents loss in polymer mass. (B) CapZ and Aip1 depolymerize actin at differing rates and to different extents. Pre-polymerized actin was treated with 2 µM cofilin +/− 0.2 µM Aip1 or CapZ. Actin depolymerizes initially in the presence of cofilin alone as described (blue line). In the presence of Aip1 (pink line), the reaction proceeds roughly 6x faster, but to the same extent as cofilin alone. 0.2 µM CapZ cause the reaction to proceed at roughly the same rate as cofilin alone but almost completely to monomer. (C, D) FRET assays to determine the critical concentration (Cc) of actin as a function of cofilin, CapZ or Aip1. Fluorescence intensity in the presence of polymerizing and non-polymerizing conditions was measured at various actin concentrations and the equation [E]= Emax* [(actin-Cc)/actin] was used to fit the data. The x-intercept represents the critical concentration. In (C), the dark grey line shows the condition actin+ cofilin, and in the presence of 1 µM cofilin alone, the critical concentration was only moderately raised (from 0.2 to 0.36). The addition of 0.1 µM Aip1 (light grey) did not significantly affect the critical concentration, however (D) the addition of 0.1 µM CapZ to cofilin, raised the critical concentration over ten-fold to 1.73 µM. Thus, Aip1 and CapZ have differing effects on actin critical concentration in the presence of cofilin. See also figure S3.