FIGURE 2.
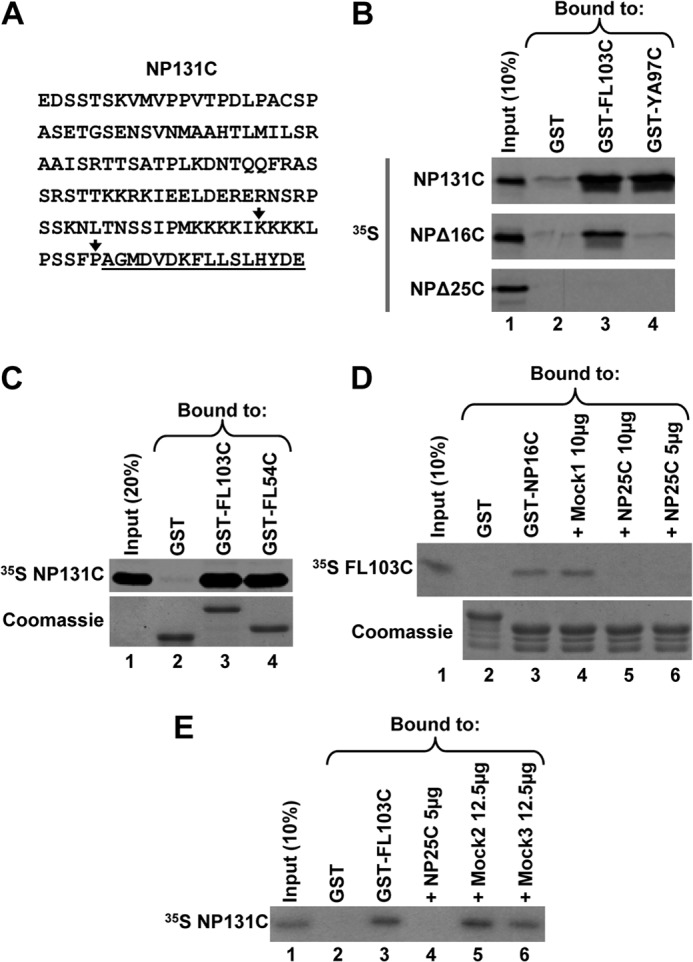
C-terminal domain shared by FLASH and YARP is sufficient for the interaction with NPAT. A, sequence of the last 131 amino acids of human NPAT (NP131C). The 16-amino acid extreme C-terminal region cloned in the yeast two-hybrid screen and capable of interacting with the C-terminal regions of FLASH and YARP is underlined. The last amino acids of the truncated NPΔ16C and NPΔ25C proteins are indicated with vertical arrows. B, GST pulldown assay to analyze in vitro interaction of 35S-labeled NP131C or its truncated versions (NPΔ16C and NPΔ25C) with the C-terminal regions of FLASH (GST-FL103C) or YARP (GST-YA97C), each fused to N-terminally positioned GST. GST alone (lane 2) was used as a negative control. The amount of each GST protein purified on glutathione beads was monitored by staining gels with Coomassie Blue and was equal in each lane (data not shown). C, GST pulldown assay to analyze the interaction of 35S-labeled NP131C with GST-FL103C or its shorter version GST-FL54C containing only the 54-amino acid region of homology with YARP (underlined in Fig. 1C). The amount of each GST protein purified on glutathione beads was monitored by staining the gel with Coomassie Blue (bottom panel). D, GST pulldown assay to analyze competition between GST-NP16C (lane 3) and Mock1 peptide (lane 4) or NP25C peptide (lanes 5 and 6) for binding 35S-labeled FL103C. GST alone (lane 2) was used as negative control. The amounts of the GST-NP16C and GST proteins purified on glutathione beads were monitored by staining the gel with Coomassie Blue (bottom panel). E, GST pulldown assay to analyze competition between 35S-labeled NP131C and NP25C peptide (lane 4) or two Mock peptides (lanes 5 and 6) for binding GST-FL103C. GST alone (lane 2) was used as negative control. The amount of each protein purified on glutathione beads was monitored by staining the gel with Coomassie Blue (data not shown).
