FIGURE 1.
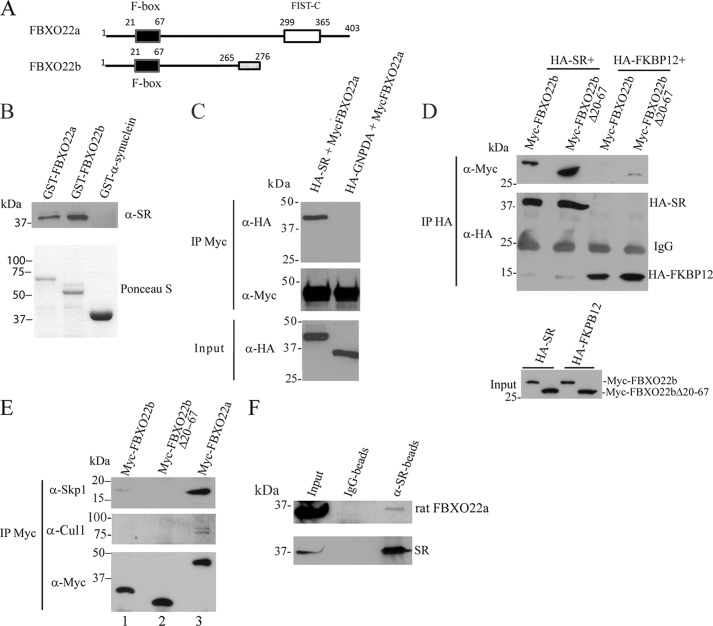
Association of SR with FBXO22 in vitro and in vivo. A, schematic of the FBXO22a and b isoforms. FBXO22b is a predicted smaller isoform that lacks the FIST C domain and whose last 11 amino acids at the C terminus are different (gray box). B, purified His-SR binds to GST-FBXO22 in vitro. His-SR (0.4 μg/ml) was incubated with glutathione-agarose beads containing 0.8 μg/ml GST-FBXO22a, 1.5 μg/ml GST-FBXO22b, or 30 μg/ml GST-α-synuclein. SR bound to the beads was monitored with anti-SR serum (1:1000) (top panel). Bottom panel, the GST-fusion proteins used in the binding experiments stained by Ponceau S. C, SR coimmunoprecipitates with FBXO22a. HEK 293 cells were cotransfected with Myc-FBXO22a and either HA-SR or HA-GNPDA (control protein), and immunoprecipitation (IP) was carried out with anti-Myc matrix. HA-SR or HA-GNPDA in the immunoprecipitate were detected with mouse anti-HA (1:1000) (top panel). Immunoprecipitated Myc-FBXO22a was detected with mouse anti-Myc (1:5000) (center panel). The bottom panel corresponds to the input (5%) probed with anti-HA. D, SR/FBXO22 interaction does not depend on the F-box domain. Myc-FBXO22b or Myc-FBXO22b Δ20–67 (lacking the F-box domain) coimmunoprecipitate with HA-SR but not with HA-FKBP12 control protein (top panel). The center panel corresponds to HA-SR and HA-FKBP12 immunoprecipitate probed with anti-HA. Levels of Myc-FBXO22a and Myc-FBXO22b in the input were monitored with polyclonal anti-Myc (1:1000) (bottom panel). The blots are representative of at least three experiments. E, coimmunoprecipitation of Myc-FBXO22a, Myc-FBXO22b, or Myc-FBXO22b Δ20–67 with endogenous Cul1 and Skp1 from HEK293 cells. Myc-FBXO22a interacts with both Skp1 and Cul1, which are the core components of the SCF complex. A much weaker interaction was observed with Myc-FBXO22b, whereas no binding was detectable with Myc-FBXO22b lacking the F-box region (Δ20–67). Top panel, coimmunoprecipitation with Skp1 monitored mouse anti-Skp1 (1:500). Center panel, coimmunoprecipitation with Cul1 monitored with mouse anti-Cul1 (1:500). F, SR and FBXO22A interact in vivo. Immunoprecipitation from rat brain homogenate with anti-SR demonstrates coimmunoprecipitation of SR to FBXO22 but not when rabbit IgG was used (B). The coimmunoprecipitation was checked using anti-FBXO22 (1:100, top panel). SR presence was verified using anti-SR serum (1:1000, bottom panel). The blots are representative of at least three experiments.