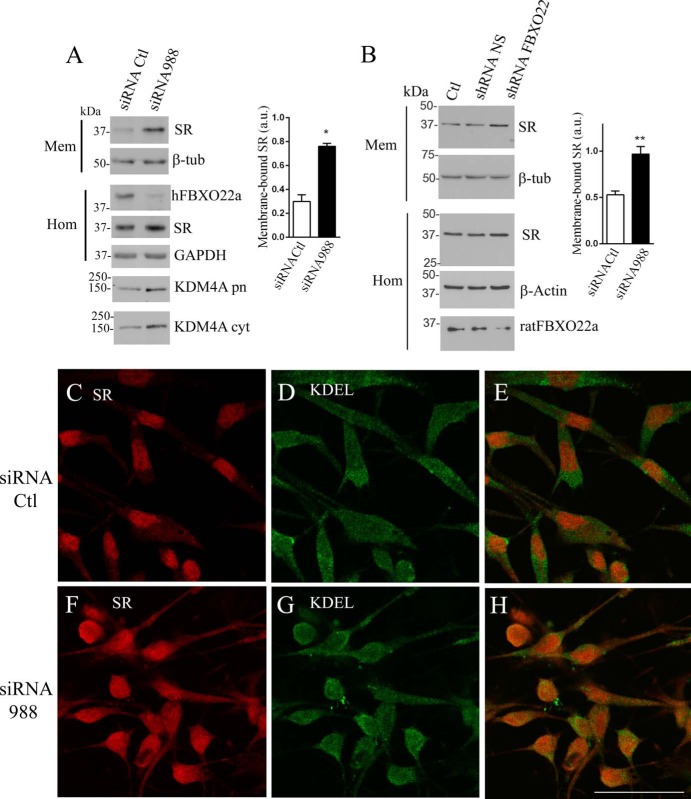
FIGURE 3.
FBXO22a primarily affects SR subcellular localization. A, siRNA-mediated knockdown of endogenous hFBXO22 expression in A172 glioblastoma cells increases endogenous SR levels in the membrane fraction. Levels of endogenous SR in the membrane fraction (Mem) increased upon hFBXO22a knockdown, whereas β-tubulin (β-tub) levels were unchanged. Knockdown of endogenous hFBXO22a monitored in the homogenate fraction (Hom) did not affect the total expression of SR or GAPDH revealed with rabbit anti-SR (1:1000) or mouse anti-GAPDH (1: 200). siRNA to hFBXO22a increased in the levels of its substrate KDM4A both in the purified nuclei fraction (KDM4 pn) and in the cytosolic fraction (KDM4 cyt), as revealed by rabbit anti-KDM4A (1:500). The graph depicts the increase in membrane-bound SR in three experiments. a.u., arbitrary units; Ctl, control. B, knockdown of endogenous rat FBXO22 expression in primary cortical neuron cultures increases membrane-bound SR levels. Primary cortical neuron cultures were infected with a lentivirus harboring shRNA to hFBXO22 or non-silencing (NS) shRNA and compared with uninfected cultures (Ctl). Subcellular fractionation reveals an increase in the levels of endogenous membrane-bound SR with no change in the β-tubulin loading control. Total levels of SR and β-actin in homogenate (Hom) were unaffected, whereas shRNA to rat FBXO22 was associated with a decrease in its expression (Hom, bottom panel). The graph depicts the increase in membrane-bound SR in three experiments. C–H, siRNA to hFBXO22 increases the extent of colocalization of endogenous SR (red) with the endoplasmic reticulum marker KDEL (green) in A172 glioblastoma cells, as analyzed by confocal laser microscopy. Control (C–E) and siRNA to hFBXO22 (F–H) were used. Scale bar = 50 μm. The panels are representative of at least three different experiments. *, p < 0.05; **, p < 0.01.