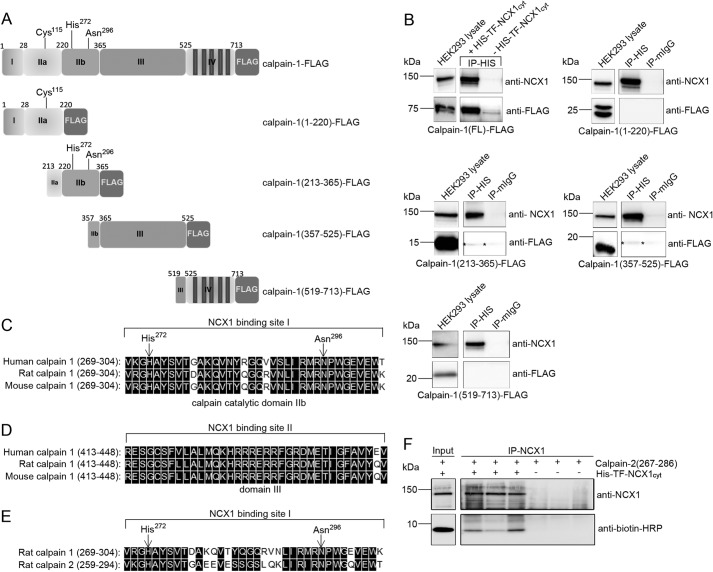
FIGURE 4.
Binding analysis in HEK293 cells and protein sequence alignments. A, schematic illustration of calpain-FLAG variants expressed in HEK293 cells. The calpain catalytic subunit consists of four domains: the N terminus (domain I), the catalytic domain (domain IIa and IIb), domain III, and domain IV (based on the calpain-2 crystal structure (51)). Cys115, His272, and Asn296 in domain IIa and IIb form the active catalytic site in the presence of Ca2+ (26). B, pull-down assays with recombinant His-TF-NCX1cyt against calpain-1-FLAG, calpain(1–220)-FLAG, calpain(213–365)-FLAG, calpain(357–525)-FLAG, and calpain(519–731)-FLAG were performed using His antibodies. Inputs and immunoprecipitates were analyzed with anti-NCX1 and anti-FLAG (n = 2–6). Mouse IgG was used as a negative control. Shown is alignment of NCX1 binding sites I and II in human, rat, and mouse calpain-1 catalytic domain IIb (C) and domain III (D), respectively. E, alignment of the NCX1 binding site in rat calpain-1 versus calpain-2 catalytic domain. His272 and Asn296 in the catalytic cleft are indicated with arrows. Black boxes, functional similar amino acids in C–E (DNA Star, Madison, WI). F, immunoprecipitation of His-TF-NCX1cyt and biotin-calpain-2(267–286) was performed with anti-NCX1. Input and immunoprecipitates were analyzed with anti-NCX1 and anti-biotin-HRP. Immunoprecipitation without His-TF-NCX1cyt was used as negative control. IP, immunoprecipitation.