FIGURE 5.
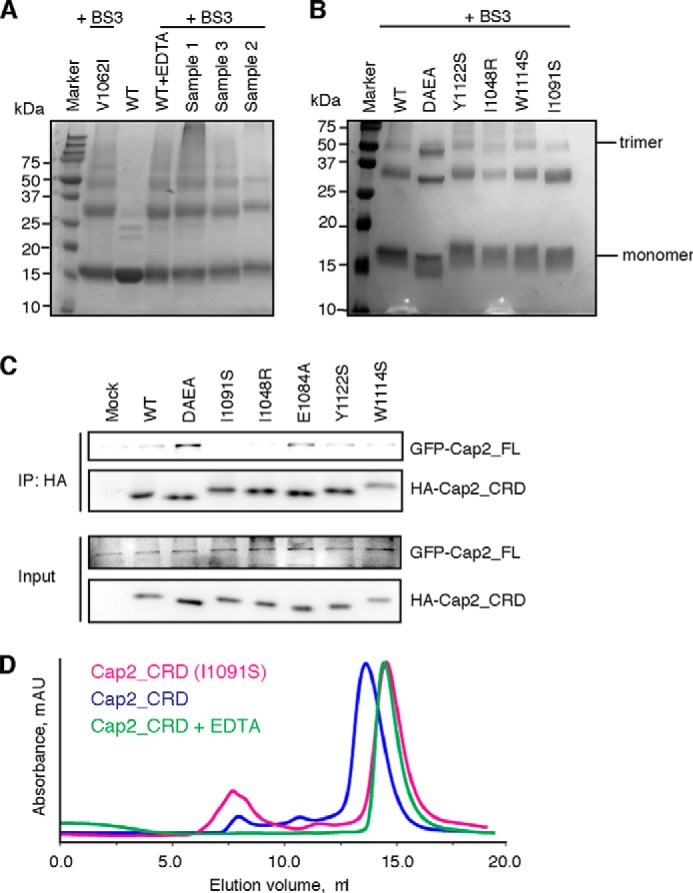
Mutations targeting trimeric interface disrupt Cap2_CRD trimer. A, BS3 cross-linking analysis showed that the trimetric bands are similar for all hCap2_CRD samples regardless of whether they contain calcium or not. Sample 1 is obtained from adding EDTA to WT Cap2_CRD. Sample 2 is obtained by further purification of sample 1 on Superdex 75 in a buffer containing 0.5 mm EDTA. Sample 3 is obtained from adding a final concentration of 5 mm CaCl2 to sample 2. V1062I is a nonsense point mutant also as a positive control. B, BS3 cross-linking analysis for mutants targeting the trimeric interface. C, HEK293T cells were co-transfected with GFP-tagged full-length Caprin-2 and HA-tagged Cap2_CRD wild type or the mutants as indicated. The lysates were then immunoprecipitated (IP) using anti-HA and then immunoblotted with anti-GFP. Compared with wild-type Cap2_CRD, I1048R and I1091S showed decreased binding affinities with the full-length Caprin-2, whereas DAEA and E1084A showed an increased binding with the full-length Caprin-2. D, the elution peak of I1091S lagged behind that of its wild-type counterpart and showed a similar position with the EDTA-treated wild-type Cap2_CRD.
