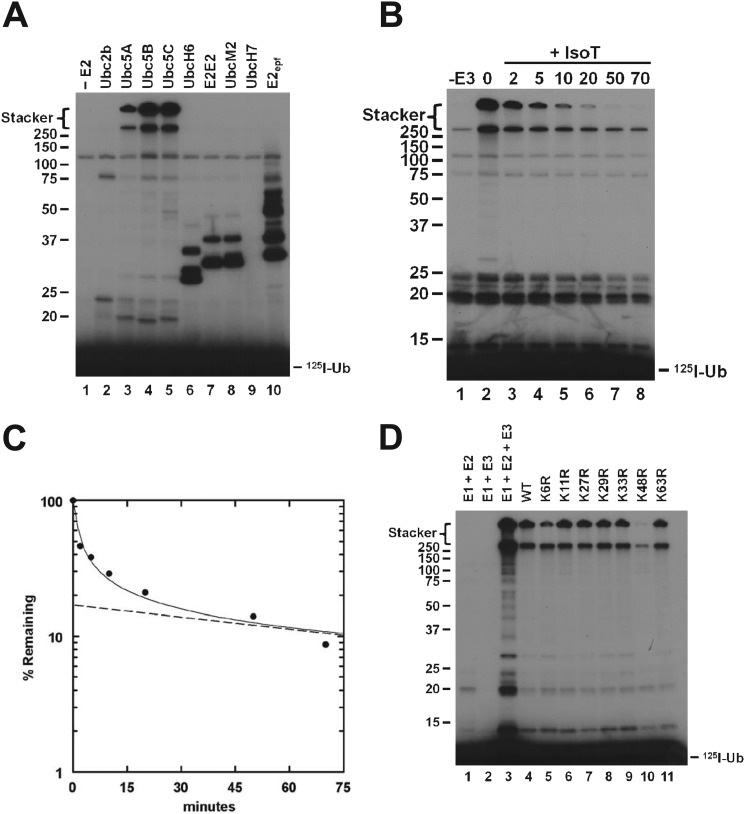
FIGURE 1.
The Ubc5 family of E2 enzymes supports IpaH9.8-catalyzed assembly of unanchored lysine 48-linked polyubiquitin chains. A, 125I-ubiquitin conjugation assays containing 5 nm IpaH9.8 were incubated for 10 min in the absence (lane 1) or presence (lanes 2–10) of a 100 nm concentration of the indicated E2 paralog and then quenched with sample buffer and resolved by 12% (w/v) SDS-PAGE under reducing conditions as described under “Materials and Methods.” The resulting gel was dried and visualized by autoradiography. B, an incubation identical to that of lane 4 of A was quenched by the addition of 8 IU of apyrase, and DTT was added to a final concentration of 10 mm to reduce all thioester bonds (0 min). Isopeptidase T was added to a final concentration of 3.8 μm by total protein, and aliquots were removed at the indicated times and quenched with sample buffer. At 50 min, additional isopeptidase T was added to a final concentration of 7.6 μm by total protein, and the reaction was allowed to continue for an additional 20 min (lane 8). The samples were resolved and visualized as in A. C, after autoradiography of the gel in B, 125I-ubiquitin conjugates larger than 25 kDa were excised and quantified by γ-counting. The percentage of 125I radioactivity remaining compared with zero time was analyzed by a semilog plot. The dashed line represents extrapolation of the limiting linear rate to t0 in order graphically to determine the fraction of IsoT refractory (anchored) versus IsoT sensitive (unanchored) chains. D, 125I-ubiquitin conjugation assays containing 5 nm IpaH9.8 were incubated for 10 min with 5 μm 125I-ubiquitin (lane 3) or 1 μm 125I-ubiquitin and a 4 μm concentration of the indicated unlabeled wild type or single lysine-to-arginine ubiquitin point mutant (lanes 4–11). Samples were resolved and visualized as in A. Mobility of relative molecular weight markers and position of the stacker gel are shown to the left. Position of free 125I-ubiquitin is shown to the right.