FIGURE 7.
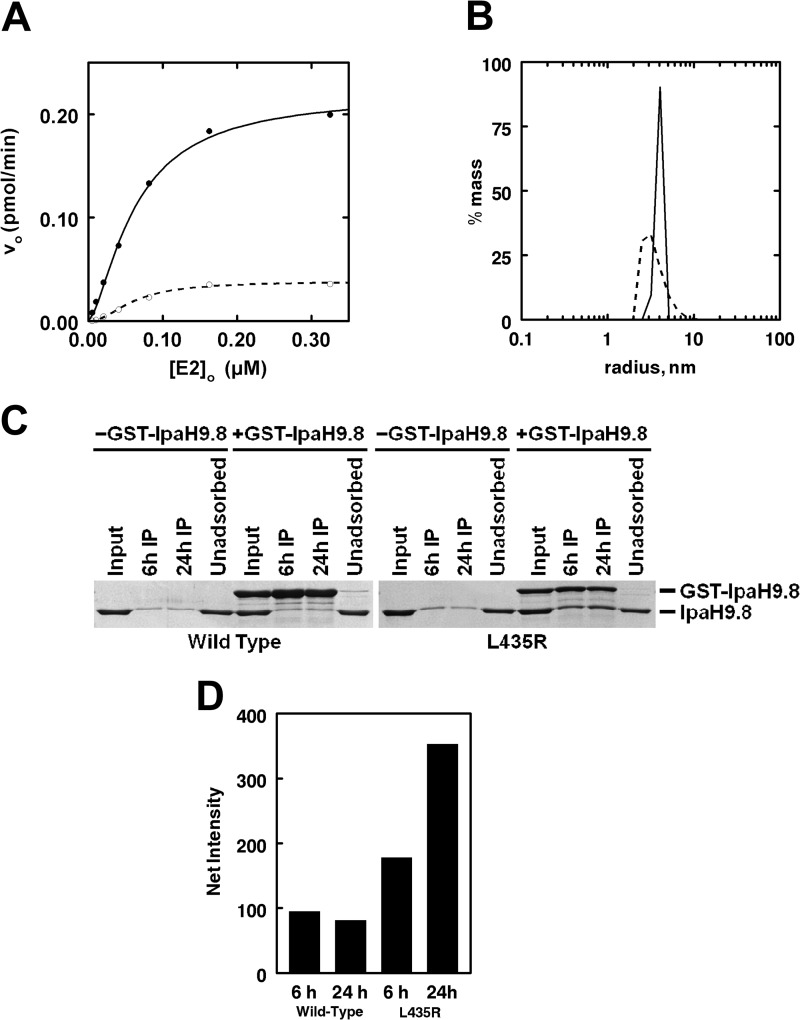
IpaH9.8 dimer formation is necessary for activity. A, initial rates of 125I-ubiquitin conjugation were determined as in Fig. 3 under E3-limiting conditions in assays containing the indicated concentrations of Ubc5B and either 2.5 nm IpaH9.8 (closed circles) or 5 nm IpaH9.8L435R (open circles). B, static light scattering analysis of 75 μm of either IpaH9.8 (solid line) or IpaH9.8L435R (dashed line). C, SDS-PAGE resolution and Coomassie Brilliant Blue staining of glutathione-Sepharose precipitation assays of reactions containing an 8.5 μm concentration of either IpaH9.8 (left) or IpaH9.8L435R (right) in the absence or presence of 8.5 μm either GST-IpaH9.8 or GST-IpaH9.8L435R, respectively. D, quantitation of the amount of IpaH9.8 or IpaH9.8L435R co-purifying with the respective GST-IpaH9.8 or GST-IpaH9.8L435R compared with background binding in the absence of the GST-fused proteins at the indicated times as analyzed by quantitative densitometry described under “Materials and Methods.”