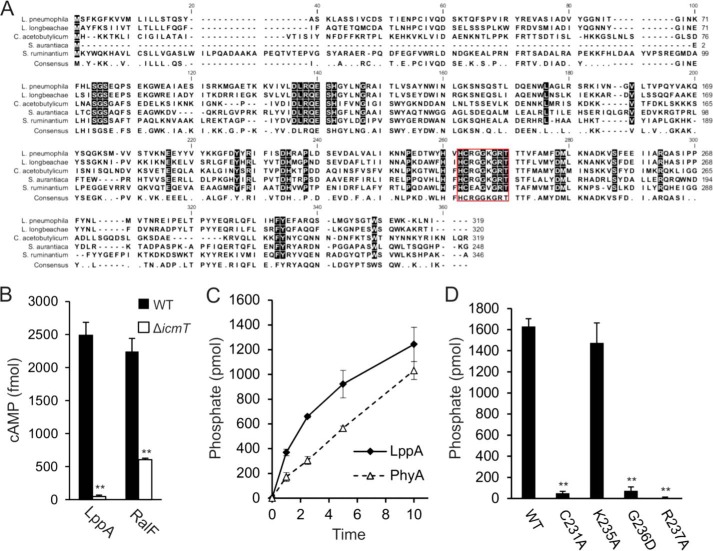
FIGURE 1.
The type IV translocated cysteine phosphatase LppA hydrolyzes phytate in vitro. A, alignment of (predicted) cysteine phytases of L. pneumophila (LppA), L. longbeachae, S. aurantica, C. acetobutylicum, and S. ruminantium (PhyA) in order of decreasing homology to LppA. The red box highlights the established or predicted P-loop catalytic active sites for LppA and PhyA as well as for the other putative phytases. B, LppA is a substrate of the Icm/Dot T4SS. RAW 264.7 macrophages were infected with wild type or ΔicmT L. pneumophila harboring pSH108 (CyaA-LppA) or pSH100 (CyaA-RalF). Levels of cAMP (mean ± S.D. (error bars), four duplicate experiments) were measured 30 min postinfection (MOI 50). C and D, hydrolysis of phytate by GST-LppAΔ1–16, GST-PhyAΔ1–16, or GST-LppAΔ1–16 point mutations of putative catalytically essential residues was measured by phosphate release as a malachite green vanadate dye complex. Reactions proceeded at 25 °C with 2 nmol of phytate and 0.5 μg of protein for up to 10 min (C) or 15 min (D). Absorbance at 620 nm was measured 20 min after termination of each reaction. The data shown are means and S.D. of triplicates (B–D) and are representative of three independent experiments (**, p < 0.005).