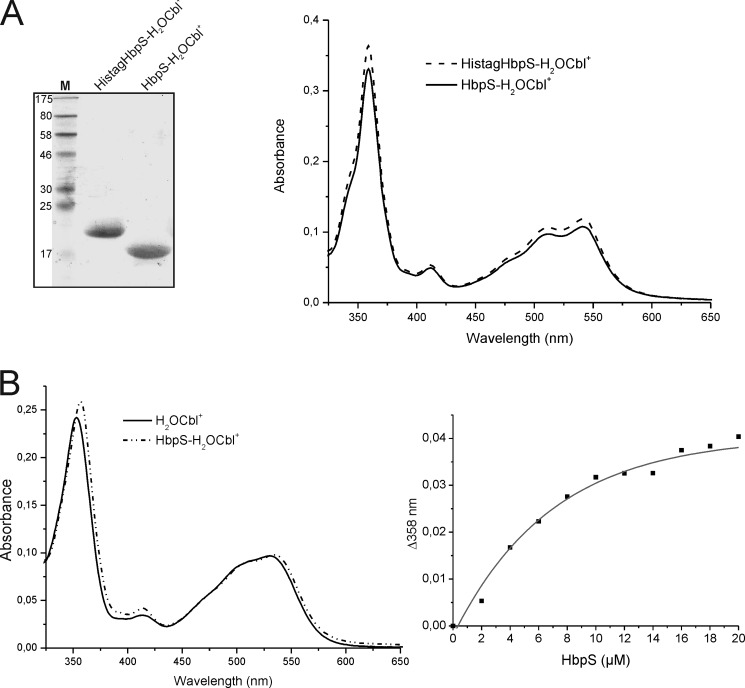
FIGURE 4.
Comparison of Cbl binding between His-tagged and His tag-free HbpS proteins and titration experiments. A, isolated His-tagged HbpS with bound H2OCbl+ (HistagHbpS-H2OCbl+) were treated with a tobacco etch virus protease. After subsequent chromatography, His tag-free HbpS with bound H2OCbl+ was obtained (HbpS-H2OCbl+). 10 μg of each protein was analyzed either SDS-PAGE (left) or UV-visible spectroscopy (right). The molecular mass (in kDa) of protein markers (lane M) is indicated (left). B, binding of H2OCbl+ by the HbpS apoprotein was monitored. Aquo-cobalamin (10 μm) was incubated with increasing concentrations (2–20 μm, with 2 μm increments) of the HbpS apoprotein for 2 h at 25 °C. UV-visible spectra of H2OCbl+ alone (black spectrum) and bound to 14 μm HbpS (dot-dashed spectrum) are shown (left). The difference absorbance at 358 nm (Δ358) was plotted against HbpS concentrations (right).