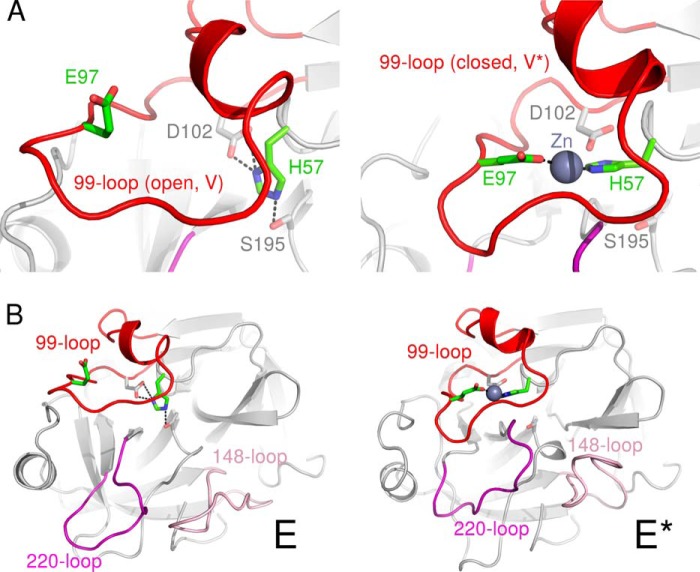
FIGURE 8.
A model of the Zn2+-induced E-E* transition in KLK2. Closeup of the 99-loop (A) and overall view of KLK2 (B) in the active E form (left) and Zn2+-inhibited E* form (right). According to this model Zn2+ binding to His-57 and Glu-97 reversibly inactivates KLK2 in two ways. First, Zn2+ disrupts the catalytic triad by dislocating His-57. In addition, the 99-, 148-, and 220-loop assume a conformation that occludes the substrate binding site. We obtained a model of the E form by grafting the well resolved 99-loop of KLK3 (PDB ID 2zch) onto the KLK2-PPACK structure. The coordinates of eKLK3 (PDB ID 1gvz) provided the basis for the model of the E* form. Additionally, we mutated Asp-97 to Glu and complemented the Zn2+ binding site by choosing an appropriate rotamer of His-57.