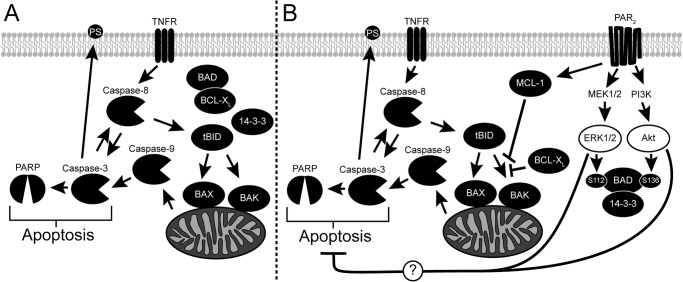
FIGURE 10.
Activation of PAR2 inhibits cytokine-induced apoptosis. A, the cytokine TNF-α activates the extrinsic apoptotic pathway by binding the TNF receptor (TNFR), which then recruits and stimulates the cleavage of caspase-8. Activated caspase-8 in turn cleaves caspase-3 as well as BID into a truncated form (tBID) to stimulate the intrinsic apoptotic pathway. Truncated BID induces conformational changes in both BAX and BAK, which oligomerize and form channels on the mitochondria's outer membrane. Cytochrome c, normally found within the mitochondria, passes through these channels into the cytosol where it stimulates the activation of caspase-9. The intrinsic and extrinsic apoptotic pathways intersect by the activation of caspases-3 by cleaved caspase-9. Many proteins vital to the normal functioning of the cell, including PARP, are digested by activated casapse-3 leading to cellular apoptosis. In healthy cells, phosphatidylserine is found on the inner leaflet of the membrane but is externalized during apoptosis in a caspase-3-dependent manner. B, activation of PAR2 stimulates both PI3K and MEK1/2, leading to the phosphorylation of BAD through downstream kinases. Phosphorylated BAD binds 14-3-3 and releases anti-apoptotic BCL-XL into the cytosol. PAR2 also increases the expression of anti-apoptotic MCL-1. Both BCL-XL and MCL-1 inhibit the oligomerization of BAX and BAK stimulated by truncated BID resulting in less mitochondrial permeability. The intrinsic apoptotic pathway is inhibited as a result leading to decreased cleavage and activation of caspase-9 and caspase-3. Cleavage of caspase-8 and PARP is also reduced due to less activated casapse-3 being present. The externalization of PS is also decreased as a result of PAR2 activation and is dependent on the activities of both MEK1/2 and PI3K, but not the presence of MCL-1 and BAD. Thus, we hypothesize that PAR2 activation blocks apoptosis through other mechanisms that are dependent on ERK1/2 and PI3K activity.