FIGURE 3.
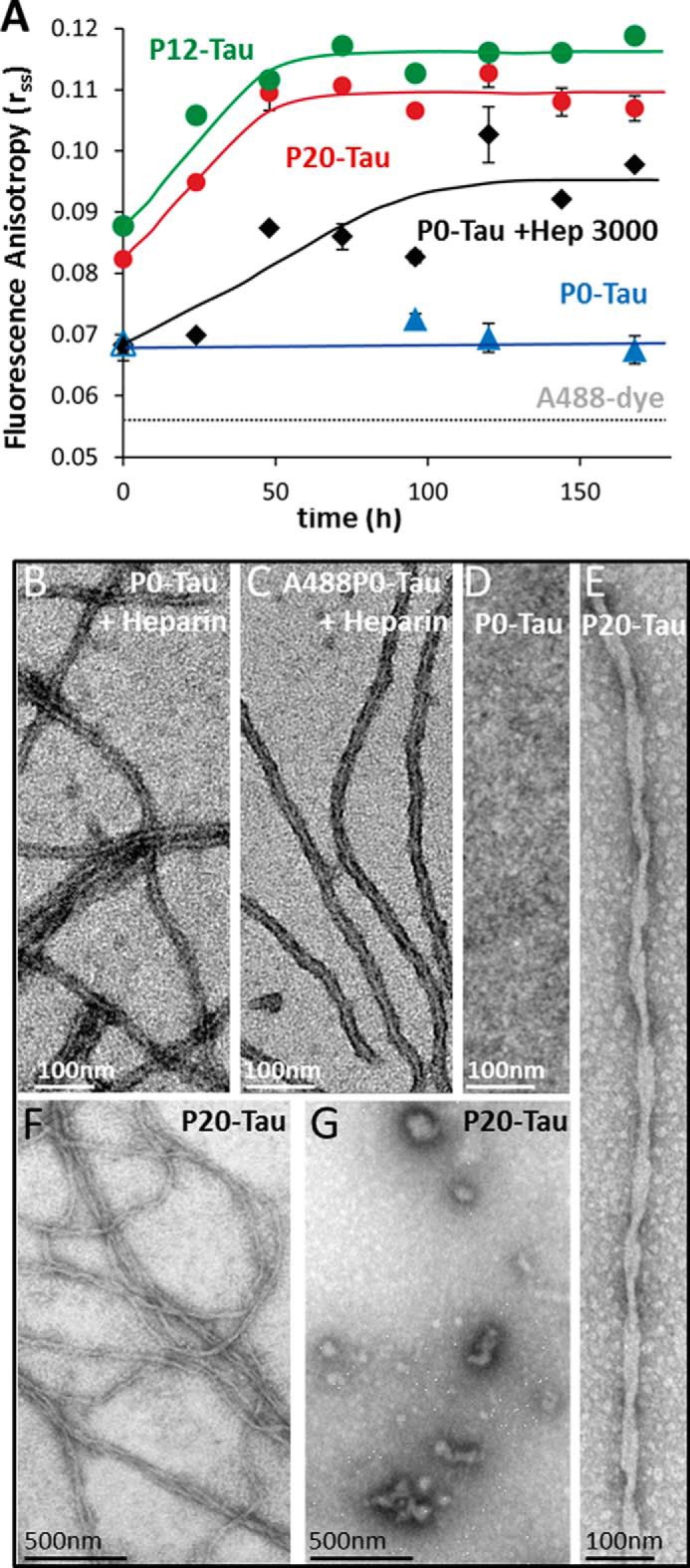
Steady-state fluorescence anisotropy (rss) demonstrates that high phosphorylation of Tau increases its ability for aggregation into oligomers and fibers. A, aggregation kinetics of highly phosphorylated Tau measured by steady-state fluorescence anisotropy. Phosphorylated P20-Tau (red dots), P12-Tau (green dots), and P0-Tau (black and blue, control) were labeled at Cys-291 and Cys-322 with Alexa dye A488. 50 μm Tau (consisting of 45 μm native and 5 μm A488-labeled-Tau) were incubated in 20 mm BES buffer, 25 mm NaCl, pH 7.4, at 37 °C for 7 days. The increase in rss reflects slower depolarization of fluorophore emission due to slower Brownian motion, indicating the increase in molecular size. In the absence of heparin, P12-Tau and P20-Tau showed an increase of rss over time, indicating faster aggregation, compared with P0-Tau, staying at the initial level. In the case of 50 μm P0-Tau, incubated in BES buffer at 37 °C with 12.5 μm heparin (black diamonds), there is a significant increase of the rss value over time, illustrating the aggregation-inducing effect of heparin. The rss value = 0.056 of the free fluorophore A488 is indicated by the dashed gray line. The lines connecting the measurement points are eye-guided lines, and the error bars indicate S.E. (n = 3). B–D, negative-stain electron micrographs of 50 μm P0-Tau (B) or 50 μm (1:1) A488-labeled P0-Tau (C), incubated with 12.5 μm heparin for 240 h, illustrates the ability of unlabeled or labeled protein to form fibers of similar appearance. By contrast, no aggregates develop without heparin (D). E–G, negative-stain electron micrographs of P20-Tau (in absence of heparin) were prepared directly after concentrating the protein to 50 μm in BES buffer. Electron micrographs demonstrate some amorphous Tau aggregates (F and G), i.e. heterogeneous oligomers and polymers (∼7% of total protein as judged by the Sarkosyl extraction method). This includes occasional twisted fibrils of aggregated P20-Tau (E) with a crossover distance of ∼80 nm, similar to AD-PHFs.
