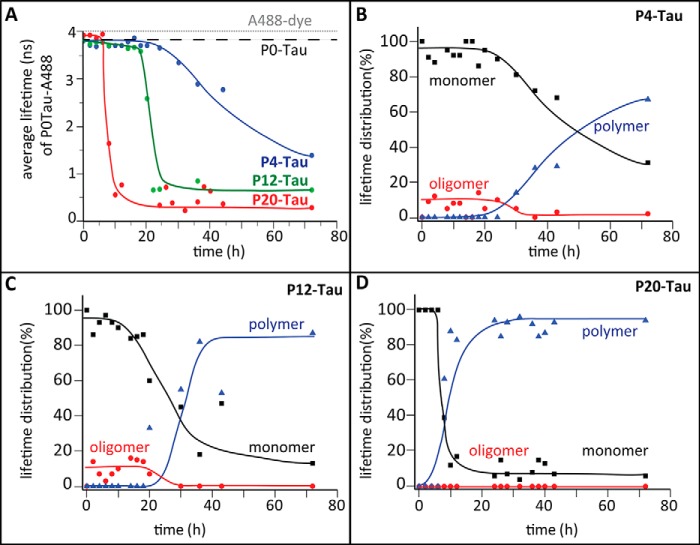
FIGURE 4.
Analysis of aggregating phosphorylated Tau by TCSPC. A, average fluorescence lifetime of the A488 probe conjugated to P0-Tau (at Cys-291 and Cys-322) was measured versus time in a (1:10) mixture with P20-Tau (red), P12-Tau (green), or P4-Tau (blue). 5 μm of each protein was incubated at 25 °C in PBS buffer, pH 7.4, containing 0.5 μm A488-P0-Tau, and measurements were taken every 120 min. The decrease of the average lifetime indicates the formation of protein aggregates due to a compaction of molecules. In contrast to the anisotropy measurements (Fig. 3), the lifetime measurement also detects dynamic molecular interactions. The average lifetime of the unbound A488-fluorophore is 4 ns (gray dashed line), and the lifetime of monomeric P0-Tau is 3.6 ns (black dashed line). P12-Tau was incubated with alkaline phosphatase, resulting in P4-Tau (blue) (de-phosphorylation was confirmed by Western blot analysis; data not shown). B–D, different lifetime components were obtained by analyzing the fractional amplitude of the average lifetime τav according to τav = τ1·α1 + τ2·α2 + τ3·α3. During the time course experiments, we grouped different lifetime components as follows: monomers (3–4-ns species, τ1), oligomers (1–3 ns; τ2), and polymers (0.1–1.0 ns; τ3). The fractional distributions of monomers (α1, black) oligomers (α2, red), and polymers (α3, blue) are represented in % versus time for P4-Tau (B), P12-Tau (C), and P20-Tau (D). All three lifetime species add up to 1 (indicated as 100%) of the fractional amplitude. Note that the presence of oligomers within the 1st h of de-phosphorylation (B) reflects the initial status of P12-Tau (compare with C), which was incubated from 0 h onward with alkaline phosphatase.