Abstract
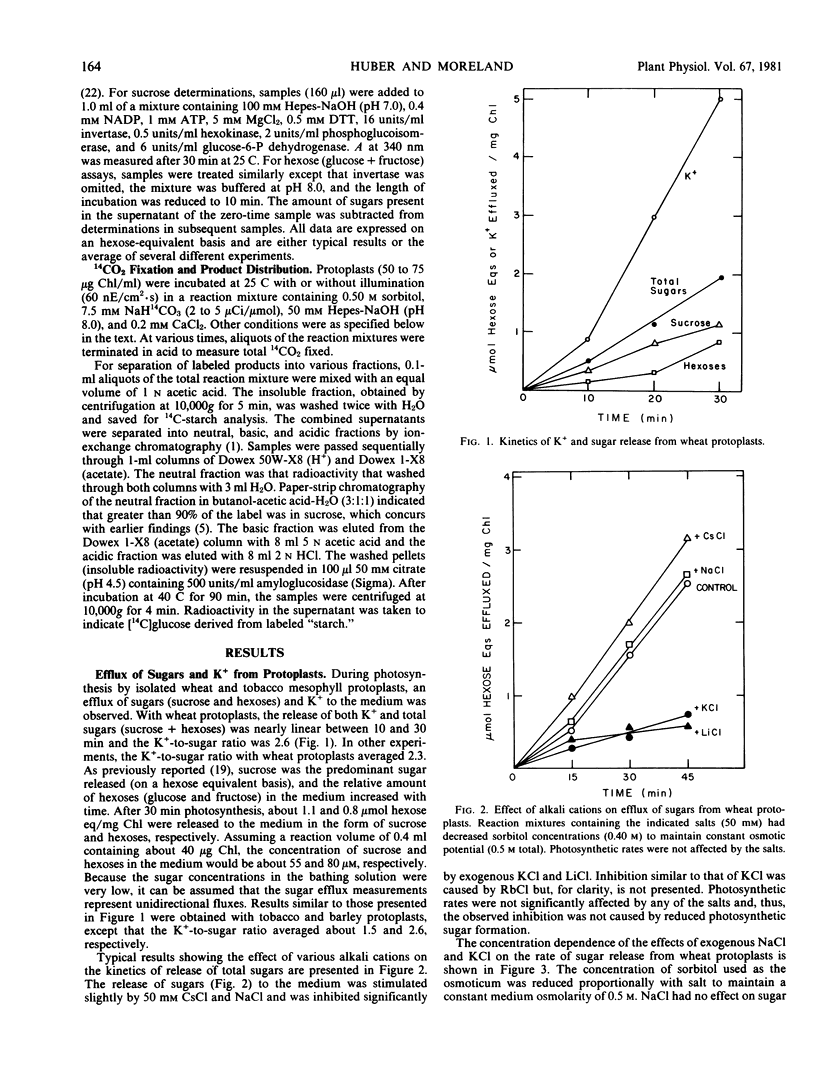
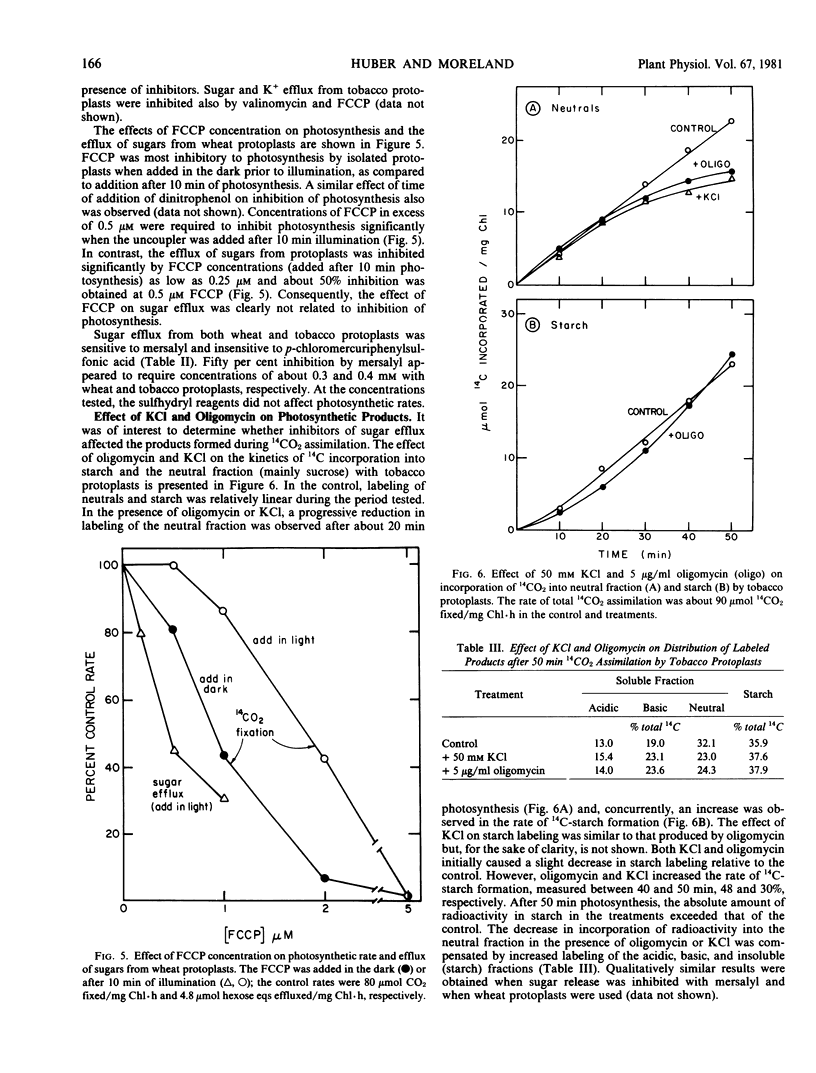
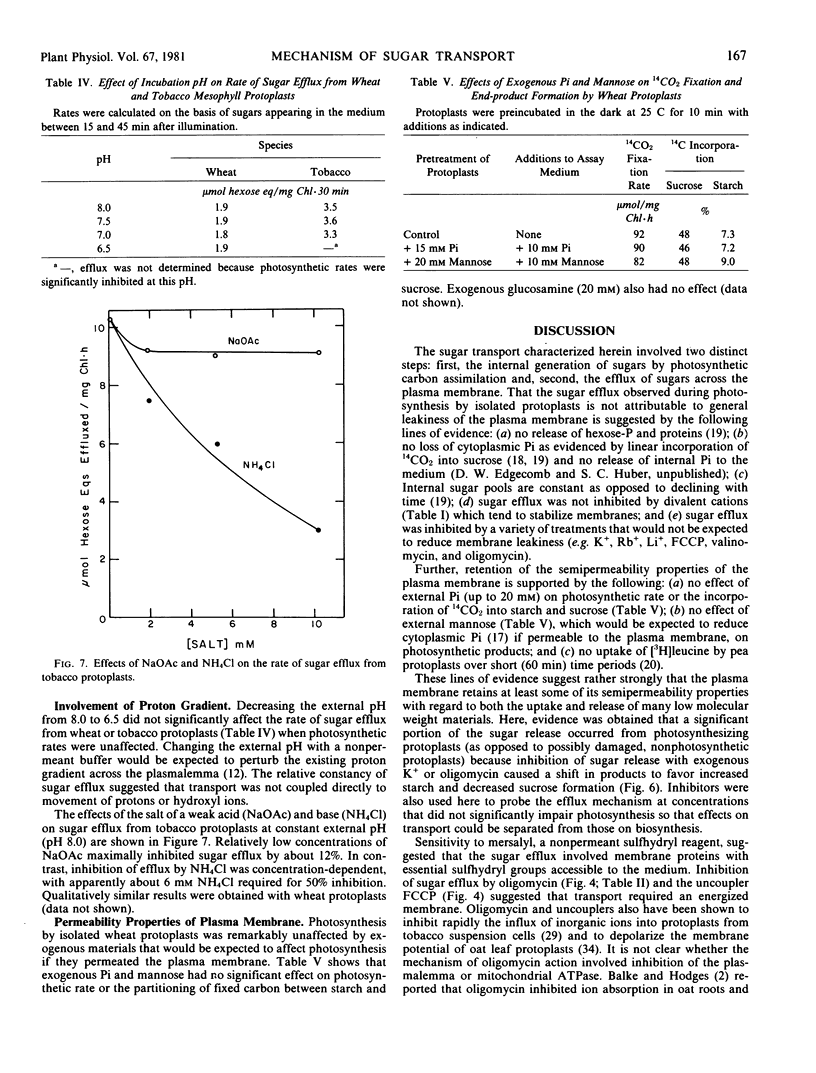
Sugars (sucrose + hexoses) produced photosynthetically by isolated mesophyll protoplasts of wheat and tobacco were effluxed across the plasma membrane (3 to 10 micromoles hexose equivalents per milligram chlorophyll per hour). The efflux was sensitive to uncouplers and oligomycin which indicated a requirement for energy. A proton gradient was probably not coupled directly to the transport because changing the proton gradient across the plasma membrane by varying the pH of the medium or by adding sodium acetate had no significant effect on the rate of sugar release.
A release of K+ was associated with sugar efflux from the protoplasts. The molar ratio of K+ to sugar varied between 1.5 and 2.5, depending on the species. Exogenous CKl, RbCl, and LiCl (50 millimolar each), but not NaCl or CsCl, significantly inhibited sugar efflux. Conditions that reduced sugar efflux (exogenous KCl, LiCl, mersalyl, or oligomycin) also reduced K+ release and caused a time-dependent reduction in photosynthetic sucrose formation and increased amino acid and starch formation. Results obtained support the postulate that a K+ symport is involved in the transport of sugar across the energized plasmalemma of photosynthetically active mesophyll cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Weer P. Intracellular pH transients induced by Co2 or NH3. Respir Physiol. 1978 Apr;33(1):41–50. doi: 10.1016/0034-5687(78)90082-8. [DOI] [PubMed] [Google Scholar]
- Doman D. C., Geiger D. R. Effect of Exogenously Supplied Foliar Potassium on Phloem Loading in Beta vulgaris L. Plant Physiol. 1979 Oct;64(4):528–533. doi: 10.1104/pp.64.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Evidence for amino Acid-h co-transport in oat coleoptiles. Plant Physiol. 1978 Jun;61(6):933–937. doi: 10.1104/pp.61.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sancho J., Sanchez A. Use of salicylic acid to measure the apparent intracellular pH in the Ehrlich ascites-tumor cell and Escherichia coli. Biochim Biophys Acta. 1978 May 4;509(1):148–158. doi: 10.1016/0005-2736(78)90015-9. [DOI] [PubMed] [Google Scholar]
- Geiger D. R., Sovonick S. A., Shock T. L., Fellows R. J. Role of free space in translocation in sugar beet. Plant Physiol. 1974 Dec;54(6):892–898. doi: 10.1104/pp.54.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Phloem Loading of Sucrose: pH Dependence and Selectivity. Plant Physiol. 1977 Apr;59(4):750–755. doi: 10.1104/pp.59.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley J. R., Hanson J. B. The action of valinomycin in uncoupling corn mitochondria. Plant Physiol. 1975 Jul;56(1):13–18. doi: 10.1104/pp.56.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Moreland D. E. Translocation: EFFLUX OF SUGARS ACROSS THE PLASMALEMMA OF MESOPHYLL PROTOPLASTS. Plant Physiol. 1980 Mar;65(3):560–562. doi: 10.1104/pp.65.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. G., Outlaw W. H., Lowry O. H. Enzymic assay of 10 to 10 moles of sucrose in plant tissues. Plant Physiol. 1977 Sep;60(3):379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Purification of enzymatically isolated mesophyll protoplasts from c(3), c(4), and crassulacean Acid metabolism plants using an aqueous dextran-polyethylene glycol two-phase system. Plant Physiol. 1973 Nov;52(5):484–490. doi: 10.1104/pp.52.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer D. W., Spanswick R. M. Correlation of Adenosine Triphosphate Levels in Chara corallina with the Activity of the Electrogenic Pump. Plant Physiol. 1979 Aug;64(2):165–168. doi: 10.1104/pp.64.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Haass D., Tanner W. Unusual features of the active hexose uptake system of Chlorella vulgaris. Biochim Biophys Acta. 1972 Jun 20;266(3):649–660. doi: 10.1016/0006-3002(72)90008-x. [DOI] [PubMed] [Google Scholar]
- Kotyk A., Ríhová L. Transport of -aminoisobutyric acid in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Nov 2;288(2):380–389. doi: 10.1016/0005-2736(72)90259-3. [DOI] [PubMed] [Google Scholar]
- Mettler I. J., Leonard R. T. Ion transport in isolated protoplasts from tobacco suspension cells: I. General characteristics. Plant Physiol. 1979 Jan;63(1):183–190. doi: 10.1104/pp.63.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt S. G. Ammonia regulation of carbon metabolism in photosynthesizing leaf discs. Plant Physiol. 1977 Nov;60(5):739–742. doi: 10.1104/pp.60.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnam C. K. Malate and Dihydroxyacetone Phosphate-dependent Nitrate Reduction in Spinach Leaf Protoplasts. Plant Physiol. 1978 Aug;62(2):220–223. doi: 10.1104/pp.62.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Walker D. A. The site of sucrose synthesis in isolated leaf protoplasts. FEBS Lett. 1979 Nov 15;107(2):295–299. doi: 10.1016/0014-5793(79)80394-4. [DOI] [PubMed] [Google Scholar]
- Rubinstein B. Use of lipophilic cations to measure the membrane potential of oat leaf protoplasts. Plant Physiol. 1978 Dec;62(6):927–929. doi: 10.1104/pp.62.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanswick R. M. Evidence for an electrogenic ion pump in Nitella translucens. I. The effects of pH, K + , Na + , light and temperature on the membrane potential and resistance. Biochim Biophys Acta. 1972 Oct 23;288(1):73–89. doi: 10.1016/0005-2736(72)90224-6. [DOI] [PubMed] [Google Scholar]


