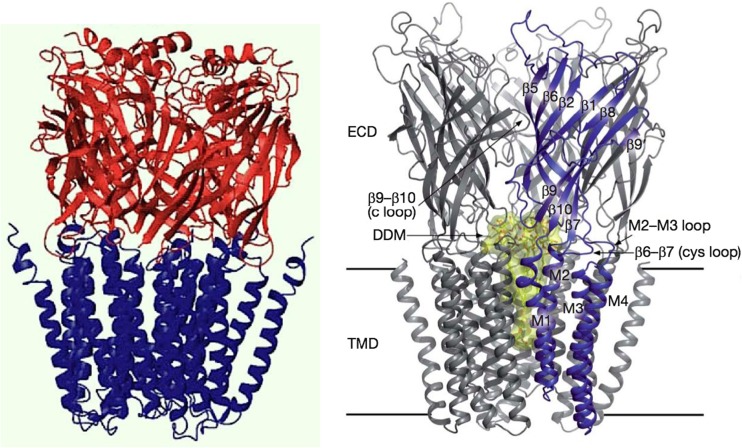
Fig. 1.
Left Model of the α7 nicotinic acetylcholine receptor (nAChR) elaborated by Taly et al. (2005) from the X-ray crystal structure of the snail acetylcholine binding protein, a homolog of the extracellular domain (Brejc et al. 2001) and the lower resolution cryo-electron microscopy data of Torpedo nAChR (Unwin 2005) for the membrane domain. From Taly et al. (2005). Right Crystal structure of a prokaryotic homolog of the nAChR from Gloeobacter violaceus [G. violaceus ligand-gated ion channel (GLIC)] in its open-channel conformation (Bocquet et al. 2009). ECD Extracellular domain, TMD transmembrane domain of four transmembrane α-helices (M1–M4) per subunit, DDM detergent dodecyl maltoside-blocked ion channel (yellow). The homology between eukaryotic and prokaryotic receptors is remarkable. From Bocquet et al (2009)