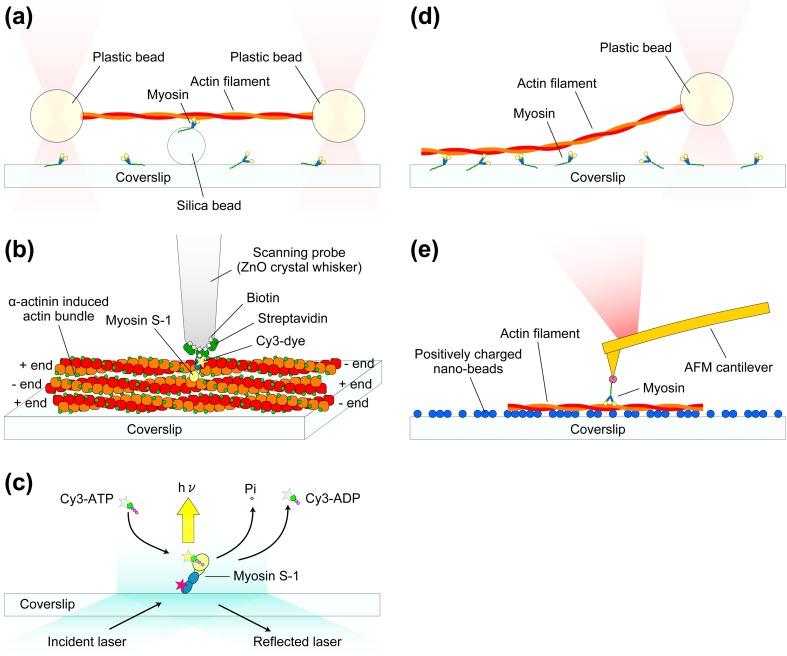
Fig. 6.
Schematics showing typical single-molecule measurement systems for studying myosin–actin interactions. a Dual-beam laser trap assay. Each end of an actin filament is attached to a plastic bead, and the beads are trapped by the focused laser beams. The trapped actin filament is brought to a single myosin molecule attached to a silica bead. b Mechanical measurement system with the use of a thin glass micro-needle as a force sensor. A ZnO crystal whisker is attached to the end of a glass micro-needle (not shown in this schematic). Fluorescently labeled single myosin S-1 is attached to the apex of the ZnO crystal whisker via a specific binding of biotin-streptavidin. The tip is brought onto actin bundles formed by α-actinin. The displacements of the S-1 resulting from the interaction with actin are monitored by the detection of glass micro-needle deflection. c TIRFM experiment for visualizing individual ATP turnovers by single myosin S-1. The light chain of S-1 molecule is labeled with Cy5 (indicated by the red star) for the confirmation of whether or not the observed ATP turnovers are those by a single S-1 molecule. Once Cy3-ATP binds to the S-1, the large Brownian motion of the Cy3-ATP stops and therefore its fluorescent spot becomes visible. d Single beam assay for the measurement of unbinding force of a single myosin–actin bond. e AFM measurement of rupture force and rupture distance of a single actin–myosin bond