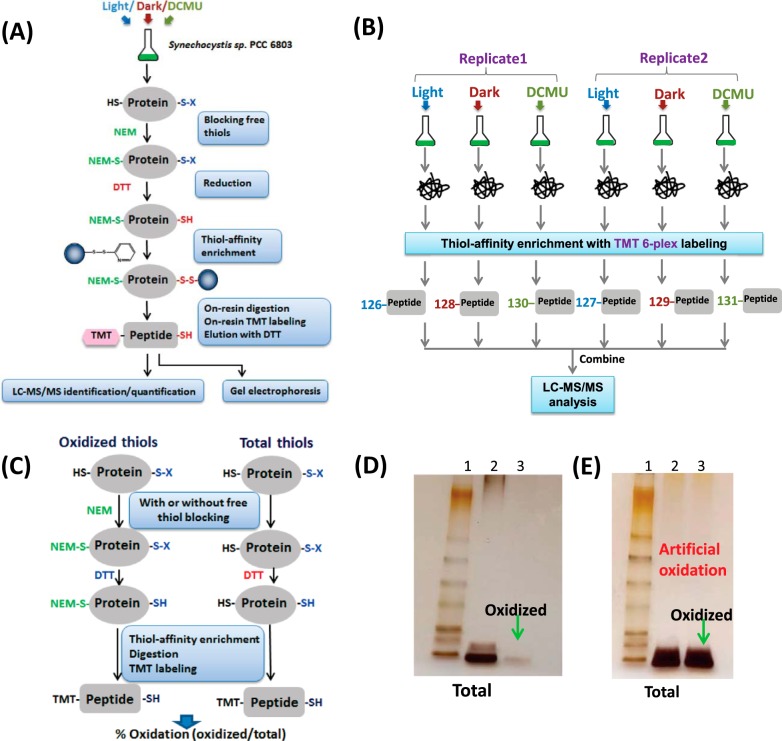
Fig. 1.
Overview of the enrichment and site-specific quantification strategy for thiol oxidation. A, enrichment and processing workflow by the resin-assisted approach. Note that S-X denotes oxidized thiols. Proteins were extracted from different conditions, and free thiols were blocked by NEM alkylation. Oxidized Cys was reduced by DTT and captured by Thiopropyl Sepharose resin. On-resin protein digestion and TMT isobaric labeling of enriched Cys-peptides were carried out and followed by DTT elution of the enriched peptides for LC-MS/MS analysis. B, six-plex quantitative strategy for profiling Cys redox dynamics. Synechocystis were cultured under continuous light, switched to dark for 2 h, or treated with 10 μm DCMU in light for 2 h. Proteins were extracted and processed to enrich the oxidized Cys-peptides accordingly, and enriched peptides were labeled with TMT reagents with different reporter tags (126–131). The six labeled samples were combined to facilitate MS/MS-based six-plex quantification. C, workflow for quantifying the percentage of reversible Cys oxidation. Equal amounts of protein samples were processed in parallel both with NEM blocking (oxidized thiols) and without NEM blocking (oxidized thiols). Total thiols (free plus reversibly oxidized thiols) were enriched in the sample reduced by DTT without NEM blocking. D, gel image of enriched total Cys-containing peptides and oxidized Cys-peptides for the samples processed with incubation with 100 mm NEM before bead-beating. Samples were from the light condition. Lane 1, standard protein ladder; Lane 2, total enriched Cys-peptides; Lane 3, enriched oxidized Cys-peptides. E, gel image of enriched total Cys-containing peptides and oxidized Cys-peptides for the samples processed with bead-beating prior to incubating with 100 mm NEM. Lane 1, standard protein ladder; Lane 2, total enriched Cys-peptides; Lane 3, enriched oxidized Cys-peptides.