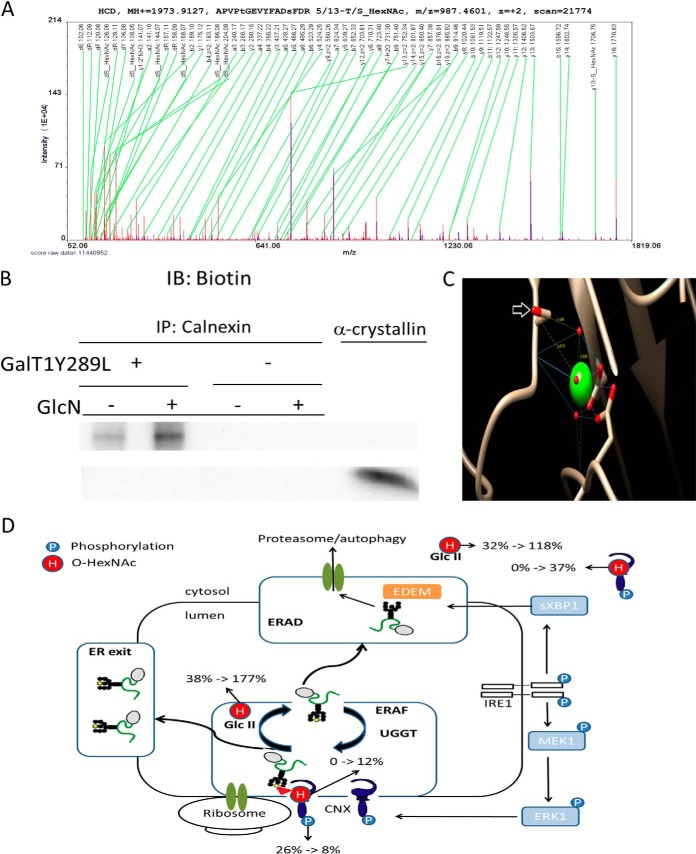
Fig. 4.
GlcN increases the O-GlcNAcylation of calnexin. A, MS/MS raw spectrum of the assigned peptide “APVPtGEVYFADsFDR 5/13-T/S_HexNAc/.” B, Purified calnexin from untreated and treated cells labeled using the Click-iT™ O-GlcNAc Enzymatic Labeling System. Detection was performed using the Click-iT™ Biotin Alkyne Detection Reagent (+). Purified calnexin (vide Supplemental Fig. S7) was subjected to the same procedure without the addition of the Gal-T1 (Y289L) enzyme (–). C, One of the mapped O-HexNAc site is in close proximity to the Ca2+ pocket in calnexin. The modified serine residues are indicated by a white arrow, and the backbone carbonyl group is involved in the metal coordination of Ca2+. D, Regulation of ER quality control under protein misfolding conditions (schematic modified from (44)). Subcellular quantitative levels of O-HexNAc and phosphorylation on calnexin. % indicates the ratio between modified and unmodified residues (in percentage); black and yellow circles represent mannose residues, and the triangle represents a glucose residue; H, O-HexNAc; P, phosphorylation; ERAF, ER-assisted folding; ERAD, ER-associated protein degradation; Glc II, glucosidase II; UGGT, UDP-glucose:glycoprotein glucosyltransferase.