Table 2.
Mean ± SD pharmacokinetic parameter estimates
| PF-05280014 | Trastuzumab-EU | Trastuzumab-US | |
|---|---|---|---|
| Subjects (n) | 34 | 35 | 32 |
| Cmax (μg ml−1) | 159 ± 26 | 174 ± 31 | 164 ± 31 |
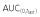 (μg ml−1 h)*
(μg ml−1 h)*
|
35700 ± 6287 | 38510 ± 6569 | 35870 ± 6878 |
| AUC(0,∞) (μg ml−1 h) | 37130 ± 6305 | 40330 ± 6994 | 37310 ± 6728 |
| CL (ml h−1 kg−1) | 0.166 ± 0.026 | 0.153 ± 0.025 | 0.166 ± 0.032 |
| Vss (ml kg−1) | 56.1 ± 8.2 | 51.7 ± 6.9 | 55.7 ± 8.8 |
| t1/2 (h) | 213 ± 42 | 220 ± 42 | 212 ± 47 |
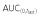 was ≥80% of corresponding AUC(0,∞) in all PK eligible subjects.
was ≥80% of corresponding AUC(0,∞) in all PK eligible subjects. 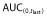 , area under the concentration–time curve from time 0 to last time point of measurable concentration; AUC(0,∞), area under the concentration–time curve from time 0 extrapolated to infinite time; CL, clearance; Cmax, maximum drug concentration; SD, standard deviation; t1/2, terminal half-life; Vss, volume of distribution at steady-state.
, area under the concentration–time curve from time 0 to last time point of measurable concentration; AUC(0,∞), area under the concentration–time curve from time 0 extrapolated to infinite time; CL, clearance; Cmax, maximum drug concentration; SD, standard deviation; t1/2, terminal half-life; Vss, volume of distribution at steady-state.
