Table 3.
Statistical comparison of pharmacokinetic exposure parameters between test and reference products
| Test | Reference | Parameter* | Geometric mean | Ratio (%)‡ | 90% CI (%) | |
|---|---|---|---|---|---|---|
| Test | Reference | |||||
| PF-05280014 | Trastuzumab-EU | Cmax | 157 | 171 | 91.49 | 85.32, 98.09 |
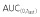 |
35210 | 38000 | 92.66 | 86.44, 99.34 | ||
| AUC(0,∞) | 36650 | 39770 | 92.15 | 86.03, 98.69 | ||
| PF-05280014 | Trastuzumab-US | Cmax | 157 | 161 | 97.41 | 90.71, 104.62 |
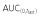 |
35210 | 35230 | 99.94 | 93.08, 107.31 | ||
| AUC(0,∞) | 36650 | 36710 | 99.83 | 93.06, 107.09 | ||
| Trastuzumab-EU | Trastuzumab-US | Cmax | 171 | 161 | 106.48 | 99.20, 114.30 |
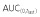 |
38000 | 35230 | 107.85 | 100.50, 115.75 | ||
| AUC(0,∞) | 39770 | 36710 | 108.34 | 101.05, 116.16 | ||
Cmax, 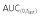 and AUC(0,∞) were in units of μg ml−1, μg ml−1 h and μg ml−1 h, respectively.
and AUC(0,∞) were in units of μg ml−1, μg ml−1 h and μg ml−1 h, respectively.
Ratio of adjusted geometric means. 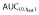 , area under the concentration–time curve from time 0 to last time point of measurable concentration; AUC(0,∞), area under the concentration–time curve from time 0 extrapolated to infinite time; CI, confidence interval; Cmax, maximum drug concentration.
, area under the concentration–time curve from time 0 to last time point of measurable concentration; AUC(0,∞), area under the concentration–time curve from time 0 extrapolated to infinite time; CI, confidence interval; Cmax, maximum drug concentration.
