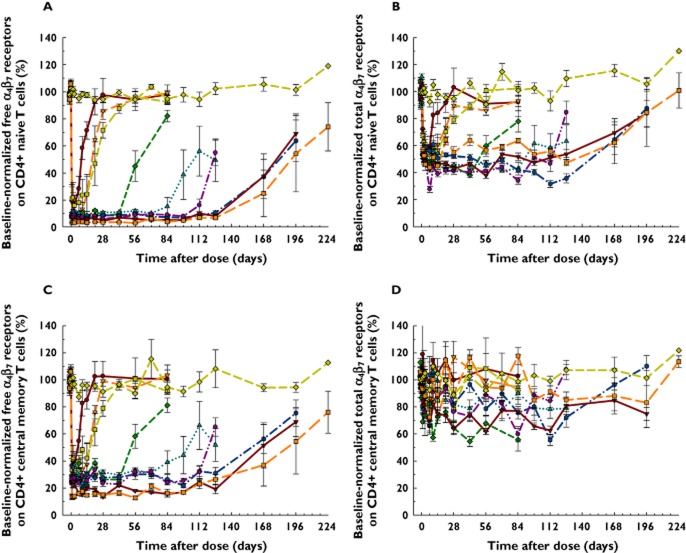
Figure 4.
Mean (SEM) percent of pre-dose baseline-normalized α4β7 receptor profiles on CD4+ naïve (A free, B total) and central memory (C free, D total) T cells after AMG 181 or placebo administration in healthy subjects. s.c. = subcutaneous; i.v. = intravenous. HV = healthy volunteers.  , Cohort 1: 0.7 mg s.c. (n = 3–4);
, Cohort 1: 0.7 mg s.c. (n = 3–4);  , Cohort 2: 2.1 mg s.c. (n = 4);
, Cohort 2: 2.1 mg s.c. (n = 4);  , Cohort 3: 7.0 mg s.c. (n = 5–6);
, Cohort 3: 7.0 mg s.c. (n = 5–6);  , Cohort 4: 21 mg s.c. (n = 5–6);
, Cohort 4: 21 mg s.c. (n = 5–6);  , Cohort 5: 70 mg s.c. (n = 4–6);
, Cohort 5: 70 mg s.c. (n = 4–6);  , Cohort 6: 210 mg s.c. (n = 4–6);
, Cohort 6: 210 mg s.c. (n = 4–6);  , Cohort 7: 70 mg i.v. (n = 5–6);
, Cohort 7: 70 mg i.v. (n = 5–6);  , Cohort 8: 210 mg i.v. (n = 4–6);
, Cohort 8: 210 mg i.v. (n = 4–6);  , Cohort 9: 420 mg i.v. (n = 4–6);
, Cohort 9: 420 mg i.v. (n = 4–6);  , placebo (n = 1–18)
, placebo (n = 1–18)