Abstract
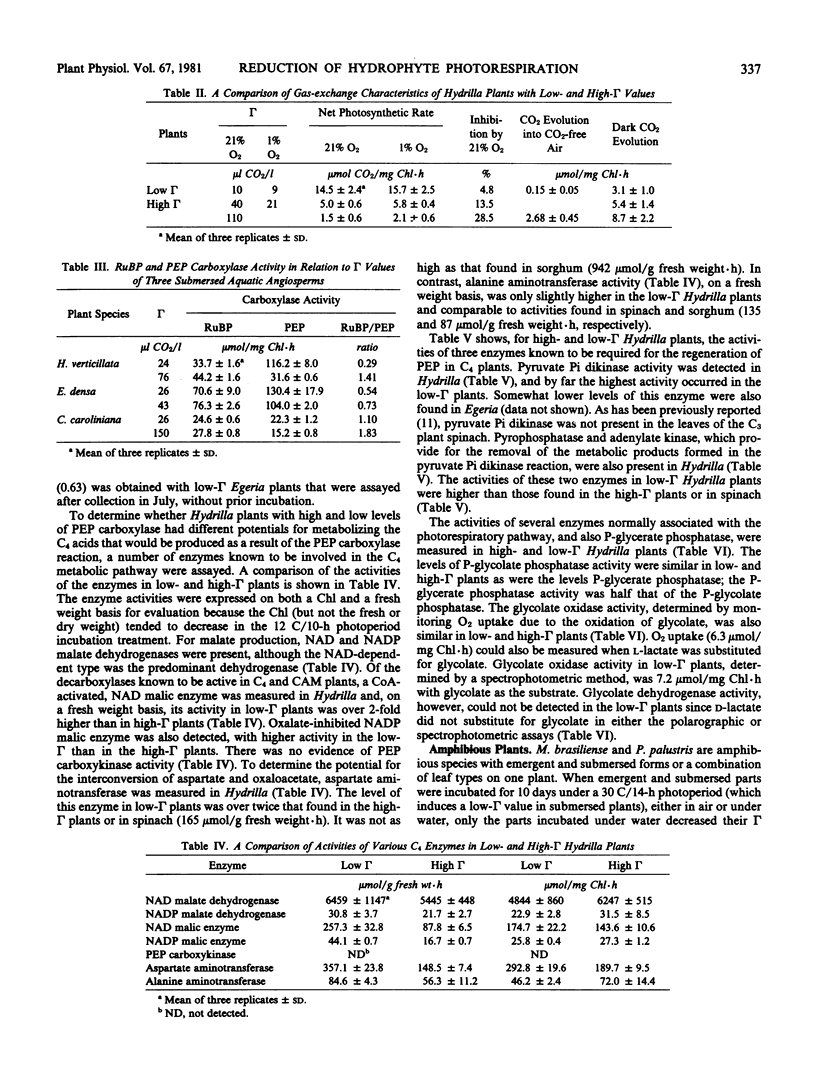
Incubation under water in a 30 C/14-hour or 12 C/10-hour photoperiod caused the CO2 compensation points of 10 aquatic macrophytes to decrease below 25 or increase above 50 microliters CO2 per liter, respectively. Submerged and aerial leaves of two amphibious angiosperms (Myriophyllum brasiliense and Proserpinaca palustris) maintained high compensation points when incubated in air but, when the submerged or aerial leaves of Proserpinaca were incubated under water, the compensation points dropped as low as 10. This suggests that, in addition to temperature and photoperiod, some factor associated with submergence regulates the compensation point of aquatic plants. In the high-compensation point plants, photorespiration, as a percentage of net photosynthesis, was equivalent to that in terrestrial C3 plants. For Hydrilla verticillata, the decreasing CO2 compensation points (110, 40, and 10) were associated with reduced photorespiration, as indicated by decreased O2 inhibition, decreased rates of CO2 evolution into CO2-free air, and increased net photosynthetic rates.
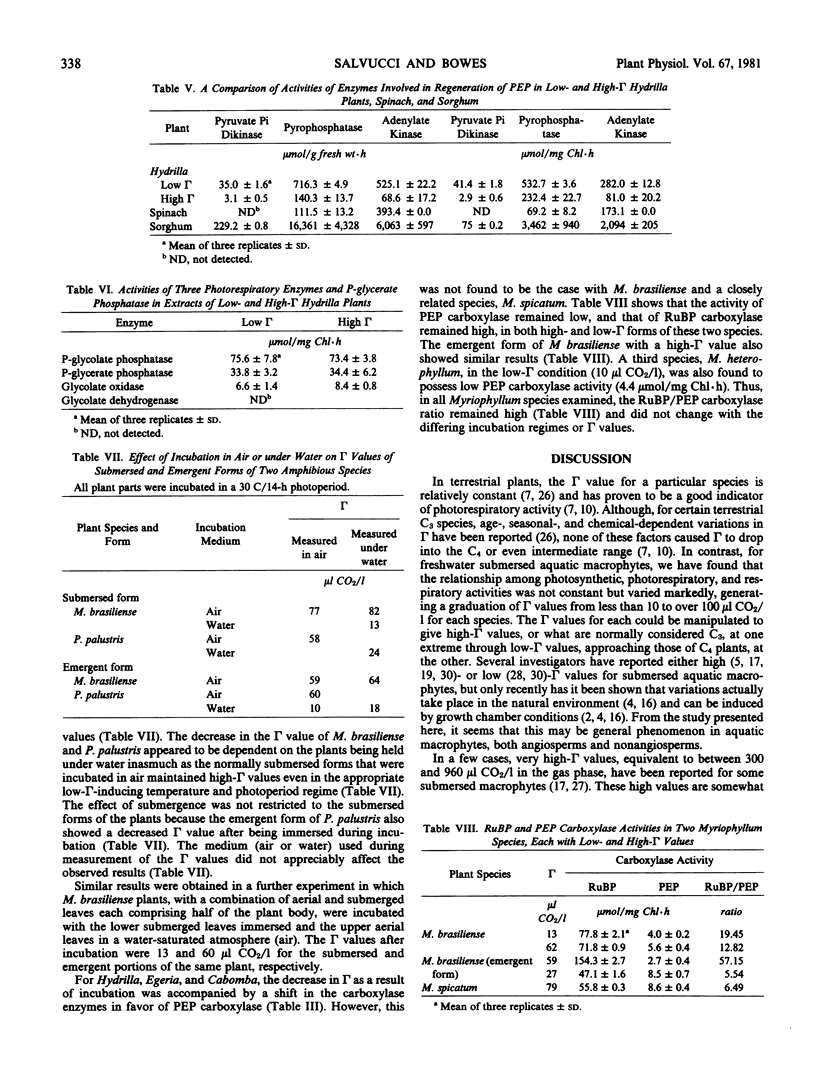
The decrease in the CO2 compensation points of Hydrilla, Egeria densa, and Cabomba caroliniana was accompanied by an increase in the activity of phosphoenolpyruvate, but not of ribulose bisphosphate, carboxylase. In Hydrilla, several C4 enzymes also increased in activity to the following levels (micromoles per gram fresh weight per hour): pyruvate Pi dikinase (35), pyrophosphatase (716), adenylate kinase (525), NAD and NADP malate dehydrogenase (6565 and 30), NAD and NADP malic enzymes (239 and 44), and aspartate and alanine aminotransferases (357 and 85), whereas glycolate oxidase (6) and phosphoglycolate and phosphoglycerate phosphatases (76 and 32) showed no change. Glycolate dehydrogenase and phosphoenolpyruvate carboxykinase were undetectable. The reduced photorespiration in these plants may be due to increased CO2 fixation via a C4 acid pathway. However, for three Myriophyllum species, some other mechanism appears operative, as phosphoenolpyruvate carboxylase was not increased in the low compensation point state, and ribulose bisphosphate carboxylase remained the predominant carboxylation enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroote D., Kennedy R. A. Photosynthesis In Elodea canadensis Michx: Four-Carbon Acid Synthesis. Plant Physiol. 1977 Jun;59(6):1133–1135. doi: 10.1104/pp.59.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick S. E., Gruber P. J., Tolbert N. E. The occurrence of glycolate dehydrogenase and glycolate oxidase in green plants: an evolutionary survey. Plant Physiol. 1973 Oct;52(4):318–323. doi: 10.1104/pp.52.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Mau S. L. Activity, location, and role of asparate aminotransferase and alanine aminotransferase isoenzymes in leaves with C4 pathway photosynthesis. Arch Biochem Biophys. 1973 May;156(1):195–206. doi: 10.1016/0003-9861(73)90357-3. [DOI] [PubMed] [Google Scholar]
- Holaday A. S., Bowes G. C(4) Acid Metabolism and Dark CO(2) Fixation in a Submersed Aquatic Macrophyte (Hydrilla verticillata). Plant Physiol. 1980 Feb;65(2):331–335. doi: 10.1104/pp.65.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and 'malic' enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1970 Sep;119(2):273–280. doi: 10.1042/bj1190273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Mazelis M., Vennesland B. Carbon Dioxide Fixation into Oxalacetate in Higher Plants. Plant Physiol. 1957 Nov;32(6):591–600. doi: 10.1104/pp.32.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall D. D., Tolbert N. E. 3-Phosphoglycerate phosphatase in plants. I. Isolation and characterization from sugarcane leaves. J Biol Chem. 1971 Sep 10;246(17):5510–5517. [PubMed] [Google Scholar]
- Smith E. W., Tolbert N. E., Ku H. S. Variables Affecting the CO(2) Compensation Point. Plant Physiol. 1976 Aug;58(2):143–146. doi: 10.1104/pp.58.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley R. A., Naylor A. W. Photosynthesis in Eurasian Watermilfoil (Myriophyllum spicatum L.). Plant Physiol. 1972 Jul;50(1):149–151. doi: 10.1104/pp.50.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van T. K., Haller W. T., Bowes G. Comparison of the photosynthetic characteristics of three submersed aquatic plants. Plant Physiol. 1976 Dec;58(6):761–768. doi: 10.1104/pp.58.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]


