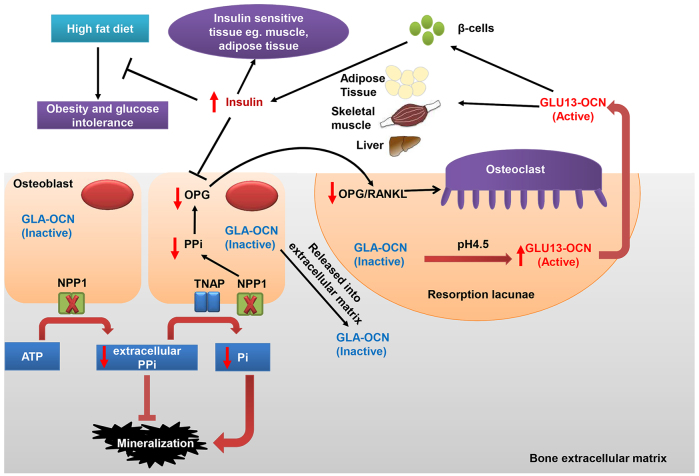
Fig. 7.
Schematic representation of the effects of NPP1 on bone mineralization, obesity and glucose intolerance. NPP1 hydrolyses ATP to generate pyrophosphate (PPi), which is a key inhibitor of mineralization. Global deletion of Enpp1 results in decreased PPi levels, leading to ectopic calcification of blood vessels and cartilage. However, in long bones such as tibiae, Enpp1 ablation leads to insufficient PPi substrate for TNAP to generate Pi for normal mineral formation. On the other hand, decreased PPi might reduce the ratio of OPG to RANKL, resulting in increased bone resorption by osteoclasts (Ribeiro et al., 2014). The acidic pH (4.5) in resorption lacunae decarboxylates osteocalcin (GLA-OCN) stored in the bone extracellular matrix to generate undercarboxylated active osteocalcin (GLU13-OCN), which stimulates insulin secretion by the β-cells of the pancreatic islets and promotes insulin sensitivity (Ferron and Lacombe, 2014). Increased insulin binds to the insulin receptor in osteoblasts and inhibits the expression of OPG, driving osteoclastic bone resorption and the further release of GLU13-OCN. Increased insulin secretion protects HFD-induced obesity and glucose intolerance, and impacts on insulin-sensitive tissues, such as muscle and adipose. Additionally, GLU13-OCN can directly stimulate energy consumption in skeletal muscle and adipose tissue and might decrease lipid accumulation in the liver (Rosen and Motyl, 2010).