In the title salt, the Pd2+ cation is located on an inversion centre and has a square-planar coordination sphere defined by four N atoms of four neutral pyrazole ligands. The two chloride anions are not coordinating to Pd2+ but are connected to the complex cations through N—H⋯Cl hydrogen bonds. C—H⋯Cl hydrogen bonds lead to a three-dimensional linkage of cations and anions.
Keywords: crystal structure, palladium(II), homoleptic metal–pyrazole complex, hydrogen bonding, solvolytic ligand degradation
Abstract
The title salt, [Pd(C3H4N2)4]Cl2, was obtained unexpectedly by the reaction of palladium(II) dichloride with equimolar amounts of 1-chloro-1-nitro-2,2,2-tris(pyrazolyl)ethane in methanol solution. The Pd2+ cation is located on an inversion centre and has a square-planar coordination sphere defined by four N atoms of four neutral pyrazole ligands. The average Pd—N distance is 2.000 (2) Å. The two chloride anions are not coordinating to Pd2+. They are connected to the complex cations through N—H⋯Cl hydrogen bonds. In addition, C—H⋯Cl hydrogen bonds are observed, leading to a three-dimensional linkage of cations and anions.
Chemical context
Transition metal complexes containing pyrazole or substituted pyrazoles as ligands are of current interest due to their supramolecular arrangements (Lumme et al., 1988 ▶; Takahashi et al., 2006 ▶; Casarin et al., 2007 ▶; Alsalme et al., 2013 ▶). In the course of an investigation on the coordination chemistry of various azolyl-nitrochloroalkanes (Zapol’skii & Kaufmann, 2008 ▶), we have previously studied the reaction of copper(II) perchlorate hexahydrate with equimolar amounts of 1-chloro-1-nitro-2,2,2-tris(pyrazolyl)ethane, Cl(NO2)CH—C(C3H3N2)3 (Fig. 1 ▶) in methanol solution (Edelmann et al., 2008 ▶). Quite unexpectedly, a complete degradation of the starting material took place during the course of this reaction. As a result, the dark-blue compound trans-bis(perchlorato)-tetrakis(pyrazole)copper(II), [Cu(C3H4N2)4(ClO4)2], was isolated. The formation of free pyrazole could only be explained by a solvolytic degradation of the starting material. This degradation must have taken place to a large extent as the isolated yield was 64% (Edelmann et al., 2008 ▶).
Figure 1.
Structure diagram of the starting material 1-chloro-1-nitro-2,2,2-tris(pyrazolyl)ethane.
We have now carried out a closely related reaction of 1-chloro-1-nitro-2,2,2-tris(pyrazolyl)ethane with palladium(II) dichloride in methanol solution. Structure determination of the yellow reaction product using X-ray analysis surprisingly again revealed the presence of a homoleptic pyrazole complex. The structure of the resultant title compound, [Pd(C3H4N2)4]Cl2 is presented here. An elemental analysis of the title compound was also in very good agreement with the composition C12H16Cl2PdN8. In this case, too, the yield was fairly high (56%), indicating a far-reaching decomposition of the starting material. Apparently, the ligand degradation of azolyl-nitrochloroalkanes in the presence of transition metal salts is a more common phenomenon than originally anticipated.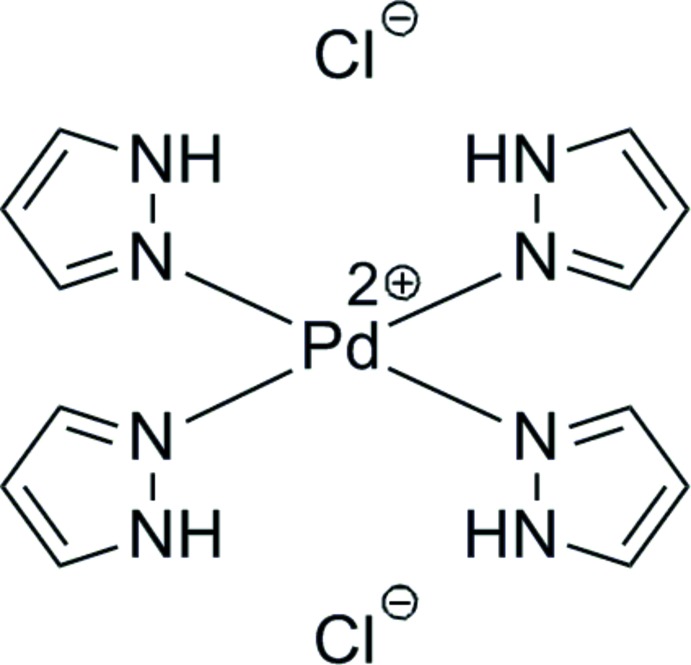
Structural commentary
In the crystal structure of the title compound, the Pd2+ ion is located on an inversion centre and is bonded to four neutral pyrazole ligands within a square-planar coordination environment (Fig. 2 ▶). The average Pd—N distance in the [Pd(pyrazole)4]2+ cation is 2.000 (2) Å. This is exactly the same value as found for the Cu—N distance in trans-bis(perchlorato)-tetrakis(pyrazole)copper(II) [2.000 (1) Å; Edelmann et al., 2008 ▶]. The two chloride anions are not coordinating to the Pd2+ cation. This is in marked contrast to the analogous copper(II) complex [Cu(pyrazole)4Cl2] (Xing et al., 2006 ▶), in which the Cu2+ ion is six-coordinated by four N atoms from four pyrazole ligands and two Cl− ions. The same octahedral coordination has also been reported for the manganese(II) analog [Mn(pyrazole)4Cl2] (Lumme, 1985 ▶).
Figure 2.
The coordination sphere of Pd2+ and the Cl− counter-ions in the title compound. Displacement ellipsoids represent the 50% probability level. [Symmetry code (A): −x +  , −y +
, −y +  , −z.]
, −z.]
Supramolecular features
In the title compound, the crystal packing is stabilized by two N—H⋯Cl hydrogen bonds (Table 1 ▶) between the complex cations and the Cl− counter-anions (Fig. 3 ▶). Weaker C—H⋯Cl hydrogen bonds are also observed, stabilizing a three-dimensional network. The crystal structures of the formally analogous complexes [M(pyrazole)4Cl2] show related features. In the structures with M = Mn and Cu and an octahedral coordination of the metal cation, the crystal structures likewise exhibit N—H⋯Cl and C—H⋯Cl hydrogen bonds which, in combination, yield three-dimensional networks.
Table 1. Hydrogen-bond geometry (, ).
| DHA | DH | HA | D A | DHA |
|---|---|---|---|---|
| N2H2NCl | 0.87(2) | 2.50(3) | 3.254(3) | 145(3) |
| N4H4NCl | 0.88(2) | 2.33(2) | 3.147(3) | 156(4) |
| C1H1Cli | 0.95 | 2.75 | 3.625(4) | 153 |
| C4H4Clii | 0.95 | 2.73 | 3.656(4) | 164 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Figure 3.
A packing diagram of the title compound. Dashed lines indicate N—H⋯Cl hydrogen-bonding interactions.
Relation with other compounds
Various closely related homoleptic metal–pyrazole complexes are known from the literature (Misra et al., 1998 ▶; Reedijk, 1969 ▶; Sastry et al., 1986 ▶). Analogous complexes of composition [M(pyrazole)4Cl2] have previously been reported for M = Mn, Fe, Co, Ni, and Cu (Daugherty & Swisher, 1968 ▶; Bagley et al., 1970 ▶; Nicholls & Warburton, 1970 ▶, 1971 ▶; Lumme, 1985 ▶; Sun et al., 2001 ▶; Xing et al., 2006 ▶). Generally, these compounds are prepared in a more straightforward manner by treatment of the transition metal dichlorides with four equivalents of pyrazole in suitable solvents such as methanol. While the analogous nickel(II) complex has been studied frequently (Daugherty & Swisher, 1968 ▶; Nicholls & Warburton, 1970 ▶), to the best of our knowledge neither the title compound nor the platinum homologue [Pt(pyrazole)4]Cl2 have ever been reported.
Synthesis and crystallization
Solid palladium(II) dichloride (0.28 g, 1.6 mmol) was added to a solution of 1-chloro-1-nitro-2,2,2-tris(pyrazolyl)ethane (0.50 g, 1.6 mmol) in methanol (100 ml). After stirring for 48 h at room temperature, a small amount of unreacted PdCl2 was removed by filtration. Crystallization from the clear filtrate at 276–279 K for 14 d afforded bright-yellow crystals of the title compound. Yield: 0.4 g (56%). Analysis calculated for C12H16Cl2PdN8: C 32.06%; H 3.59%; N 24.92%; Cl 15.77%; found: C 31.55%; H 3.38%; N 25.13%; Cl 15.25%. IR (KBr): 3090vs, 2977vs, 2371m, 1798w, 1772w, 1632w, 1518m, 1487m, 1472s, 1401m, 1367s, 1312m, 1264m, 1251m, 1209w, 1181vs, 1169m, 1139s, 1123s, 1078vs, 1052vs, 983m, 956m, 913m, 908m, 899m, 886m, 878m, 779vs, 739s, 615s, 606s cm−1.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▶. The hydrogen atoms attached to carbon were included using a riding model, with C—H = 0.95 Å, and with U iso(H) = 1.2U eq(C). The hydrogen atoms attached to nitrogen were refined with a restrained distance N—H = 0.88 (2) Å and with U iso(H) = 1.2U eq(N).
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Pd(C12H16N8)]Cl2 |
| M r | 449.63 |
| Crystal system, space group | Monoclinic, C2/c |
| Temperature (K) | 150 |
| a, b, c () | 13.797(3), 9.6560(19), 14.174(3) |
| () | 117.80(3) |
| V (3) | 1670.4(6) |
| Z | 4 |
| Radiation type | Mo K |
| (mm1) | 1.44 |
| Crystal size (mm) | 0.40 0.40 0.20 |
| Data collection | |
| Diffractometer | Stoe IPDS 2T |
| Absorption correction | Multi-scan (Blessing, 1995 ▶) |
| T min, T max | 0.562, 0.750 |
| No. of measured, independent and observed [I > 2(I)] reflections | 7700, 2253, 2030 |
| R int | 0.054 |
| (sin /)max (1) | 0.686 |
| Refinement | |
| R[F 2 > 2(F 2)], wR(F 2), S | 0.039, 0.094, 1.12 |
| No. of reflections | 2253 |
| No. of parameters | 112 |
| No. of restraints | 2 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| max, min (e 3) | 1.79, 1.73 |
Supplementary Material
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S160053681402460X/wm5084sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681402460X/wm5084Isup2.hkl
CCDC reference: 1033485
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
Financial support of this work by the Otto-von-Guericke-Universität Magdeburg is gratefully acknowledged.
supplementary crystallographic information
Crystal data
| [Pd(C12H16N8)]Cl2 | F(000) = 896 |
| Mr = 449.63 | 449.6 |
| Monoclinic, C2/c | Dx = 1.788 Mg m−3 |
| Hall symbol: -C 2yc | Mo Kα radiation, λ = 0.71073 Å |
| a = 13.797 (3) Å | Cell parameters from 15423 reflections |
| b = 9.6560 (19) Å | θ = 2.7–29.6° |
| c = 14.174 (3) Å | µ = 1.44 mm−1 |
| β = 117.80 (3)° | T = 150 K |
| V = 1670.4 (6) Å3 | Prism, yellow |
| Z = 4 | 0.40 × 0.40 × 0.20 mm |
Data collection
| Stoe IPDS-2T diffractometer | 2253 independent reflections |
| Radiation source: fine-focus sealed tube | 2030 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.054 |
| Detector resolution: 6.67 pixels mm-1 | θmax = 29.2°, θmin = 2.7° |
| ω–scans | h = −18→18 |
| Absorption correction: multi-scan (Blessing, 1995) | k = −13→13 |
| Tmin = 0.562, Tmax = 0.750 | l = −17→19 |
| 7700 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.039 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.094 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.12 | w = 1/[σ2(Fo2) + (0.049P)2 + 4.2172P] where P = (Fo2 + 2Fc2)/3 |
| 2253 reflections | (Δ/σ)max < 0.001 |
| 112 parameters | Δρmax = 1.79 e Å−3 |
| 2 restraints | Δρmin = −1.73 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Pd | 0.2500 | 0.2500 | 0.0000 | 0.01539 (11) | |
| Cl | 0.09118 (6) | −0.02409 (9) | −0.06859 (6) | 0.02881 (17) | |
| N1 | 0.2363 (2) | 0.2373 (2) | 0.1343 (2) | 0.0190 (5) | |
| N2 | 0.1606 (2) | 0.1607 (3) | 0.1453 (2) | 0.0225 (5) | |
| H2N | 0.116 (3) | 0.109 (4) | 0.093 (2) | 0.027* | |
| N3 | 0.36913 (18) | 0.1080 (3) | 0.05645 (18) | 0.0189 (4) | |
| N4 | 0.3490 (2) | −0.0279 (3) | 0.0486 (2) | 0.0234 (5) | |
| H4N | 0.2803 (18) | −0.054 (5) | 0.015 (2) | 0.028* | |
| C1 | 0.1706 (3) | 0.1749 (3) | 0.2435 (2) | 0.0269 (6) | |
| H1 | 0.1268 | 0.1311 | 0.2702 | 0.032* | |
| C2 | 0.2560 (3) | 0.2644 (3) | 0.2984 (3) | 0.0284 (7) | |
| H2 | 0.2832 | 0.2944 | 0.3702 | 0.034* | |
| C3 | 0.2943 (2) | 0.3019 (3) | 0.2264 (2) | 0.0234 (5) | |
| H3 | 0.3529 | 0.3642 | 0.2412 | 0.028* | |
| C4 | 0.4419 (3) | −0.0999 (4) | 0.0945 (3) | 0.0302 (6) | |
| H4 | 0.4484 | −0.1979 | 0.0986 | 0.036* | |
| C5 | 0.5262 (3) | −0.0071 (4) | 0.1346 (3) | 0.0316 (7) | |
| H5 | 0.6023 | −0.0271 | 0.1721 | 0.038* | |
| C6 | 0.4771 (2) | 0.1239 (3) | 0.1089 (2) | 0.0267 (6) | |
| H6 | 0.5148 | 0.2099 | 0.1263 | 0.032* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Pd | 0.01373 (15) | 0.01505 (16) | 0.01795 (15) | 0.00207 (9) | 0.00784 (11) | 0.00096 (9) |
| Cl | 0.0255 (3) | 0.0302 (4) | 0.0336 (3) | −0.0083 (3) | 0.0162 (3) | −0.0104 (3) |
| N1 | 0.0183 (10) | 0.0186 (12) | 0.0216 (10) | 0.0018 (8) | 0.0105 (9) | 0.0021 (8) |
| N2 | 0.0227 (11) | 0.0210 (12) | 0.0275 (11) | −0.0027 (9) | 0.0149 (9) | −0.0005 (9) |
| N3 | 0.0160 (10) | 0.0175 (11) | 0.0222 (10) | 0.0035 (8) | 0.0080 (8) | 0.0017 (8) |
| N4 | 0.0199 (10) | 0.0173 (11) | 0.0329 (12) | 0.0025 (9) | 0.0122 (9) | 0.0016 (10) |
| C1 | 0.0286 (14) | 0.0259 (16) | 0.0322 (14) | 0.0049 (12) | 0.0192 (12) | 0.0057 (12) |
| C2 | 0.0299 (15) | 0.0337 (18) | 0.0213 (13) | 0.0118 (12) | 0.0117 (12) | 0.0023 (11) |
| C3 | 0.0202 (12) | 0.0248 (14) | 0.0218 (12) | 0.0037 (11) | 0.0069 (10) | −0.0016 (11) |
| C4 | 0.0303 (14) | 0.0252 (16) | 0.0391 (16) | 0.0093 (12) | 0.0195 (13) | 0.0067 (13) |
| C5 | 0.0216 (13) | 0.0325 (18) | 0.0392 (16) | 0.0134 (12) | 0.0128 (12) | 0.0094 (13) |
| C6 | 0.0158 (11) | 0.0238 (15) | 0.0342 (15) | 0.0005 (10) | 0.0063 (11) | −0.0009 (12) |
Geometric parameters (Å, º)
| Pd—N3 | 1.999 (2) | N4—H4N | 0.875 (19) |
| Pd—N3i | 1.999 (2) | C1—C2 | 1.372 (5) |
| Pd—N1i | 2.002 (3) | C1—H1 | 0.9500 |
| Pd—N1 | 2.002 (3) | C2—C3 | 1.399 (4) |
| N1—C3 | 1.327 (4) | C2—H2 | 0.9500 |
| N1—N2 | 1.347 (3) | C3—H3 | 0.9500 |
| N2—C1 | 1.340 (4) | C4—C5 | 1.365 (5) |
| N2—H2N | 0.869 (18) | C4—H4 | 0.9500 |
| N3—C6 | 1.327 (4) | C5—C6 | 1.400 (4) |
| N3—N4 | 1.336 (3) | C5—H5 | 0.9500 |
| N4—C4 | 1.332 (4) | C6—H6 | 0.9500 |
| N3—Pd—N3i | 180.00 (11) | N2—C1—C2 | 107.4 (3) |
| N3—Pd—N1i | 89.89 (10) | N2—C1—H1 | 126.3 |
| N3i—Pd—N1i | 90.11 (10) | C2—C1—H1 | 126.3 |
| N3—Pd—N1 | 90.11 (10) | C1—C2—C3 | 105.4 (3) |
| N3i—Pd—N1 | 89.89 (10) | C1—C2—H2 | 127.3 |
| N1i—Pd—N1 | 180.0 (2) | C3—C2—H2 | 127.3 |
| C3—N1—N2 | 106.7 (2) | N1—C3—C2 | 109.7 (3) |
| C3—N1—Pd | 128.7 (2) | N1—C3—H3 | 125.2 |
| N2—N1—Pd | 124.51 (19) | C2—C3—H3 | 125.2 |
| C1—N2—N1 | 110.8 (3) | N4—C4—C5 | 107.4 (3) |
| C1—N2—H2N | 130 (3) | N4—C4—H4 | 126.3 |
| N1—N2—H2N | 119 (3) | C5—C4—H4 | 126.3 |
| C6—N3—N4 | 107.2 (2) | C4—C5—C6 | 105.7 (3) |
| C6—N3—Pd | 130.1 (2) | C4—C5—H5 | 127.2 |
| N4—N3—Pd | 122.71 (18) | C6—C5—H5 | 127.2 |
| C4—N4—N3 | 110.9 (3) | N3—C6—C5 | 108.8 (3) |
| C4—N4—H4N | 132 (3) | N3—C6—H6 | 125.6 |
| N3—N4—H4N | 117 (3) | C5—C6—H6 | 125.6 |
| N3—Pd—N1—C3 | 84.2 (3) | N1—Pd—N3—N4 | 83.8 (2) |
| N3i—Pd—N1—C3 | −95.8 (3) | C6—N3—N4—C4 | −0.4 (3) |
| N1i—Pd—N1—C3 | −138 (100) | Pd—N3—N4—C4 | −178.5 (2) |
| N3—Pd—N1—N2 | −97.9 (2) | N1—N2—C1—C2 | 0.1 (3) |
| N3i—Pd—N1—N2 | 82.1 (2) | N2—C1—C2—C3 | 0.4 (3) |
| N1i—Pd—N1—N2 | 40 (100) | N2—N1—C3—C2 | 0.9 (3) |
| C3—N1—N2—C1 | −0.6 (3) | Pd—N1—C3—C2 | 179.0 (2) |
| Pd—N1—N2—C1 | −178.9 (2) | C1—C2—C3—N1 | −0.8 (3) |
| N3i—Pd—N3—C6 | 127 (100) | N3—N4—C4—C5 | 0.4 (4) |
| N1i—Pd—N3—C6 | 86.1 (3) | N4—C4—C5—C6 | −0.3 (4) |
| N1—Pd—N3—C6 | −93.9 (3) | N4—N3—C6—C5 | 0.2 (4) |
| N3i—Pd—N3—N4 | −55 (100) | Pd—N3—C6—C5 | 178.1 (2) |
| N1i—Pd—N3—N4 | −96.2 (2) | C4—C5—C6—N3 | 0.1 (4) |
Symmetry code: (i) −x+1/2, −y+1/2, −z.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2N···Cl | 0.87 (2) | 2.50 (3) | 3.254 (3) | 145 (3) |
| N4—H4N···Cl | 0.88 (2) | 2.33 (2) | 3.147 (3) | 156 (4) |
| C1—H1···Clii | 0.95 | 2.75 | 3.625 (4) | 153 |
| C4—H4···Cliii | 0.95 | 2.73 | 3.656 (4) | 164 |
Symmetry codes: (ii) x, −y, z+1/2; (iii) −x+1/2, −y−1/2, −z.
References
- Alsalme, A., Al-Farhan, K., Ghazzali, M., Khair, M., Khan, R. A. & Reedijk, J. (2013). Inorg. Chim. Acta, 407, 7–10.
- Bagley, M. J., Nicholls, D. & Warburton, B. A. (1970). J. Chem. Soc. A, pp. 2694–2697.
- Blessing, R. H. (1995). Acta Cryst. A51, 33–38. [DOI] [PubMed]
- Casarin, M., Cingolani, A., Di Nicola, C., Falcomer, D., Monari, M., Pandolfo, L. & Pettinari, C. (2007). Cryst. Growth Des. 7, 676–685.
- Daugherty, N. A. & Swisher, J. H. (1968). Inorg. Chem. 7, 1651–1653.
- Edelmann, F. T., Kaufmann, D. E., Blaurock, S., Wagner, T. & Zapol’skii, V. (2008). Acta Cryst. E64, m1315. [DOI] [PMC free article] [PubMed]
- Lumme, P. O. (1985). Thermochim. Acta, 86, 101–108.
- Lumme, P. O., Lindell, E. & Mutikainen, I. (1988). Acta Cryst. C44, 967–970.
- Misra, B. N., Kripal, R. & Narayan, A. (1998). Indian J. Pure Appl. Phys. 36, 412–414.
- Nicholls, D. & Warburton, B. A. (1970). J. Inorg. Nucl. Chem. 32, 3871–3874.
- Nicholls, D. & Warburton, B. A. (1971). J. Inorg. Nucl. Chem. 33, 1041–1045.
- Reedijk, J. (1969). Recl Trav. Chim. Pays Bas, 88, 1451–1470.
- Sastry, B. A., Balaiah, B., Reddy, K. V. G., Madhu, B., Ponticelli, G., Massacssi, M. & Puggioni, G. (1986). Indian J. Pure Appl. Phys. 24, 460–462.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Stoe (2008). X-AREA and X-RED32. Stoe & Cie, Darmstadt, Germany.
- Sun, Y.-J., Cheng, P., Yan, S.-P., Liao, D.-Z., Jiang, Z.-H. & Shen, P.-W. (2001). J. Mol. Struct. 597, 191–198.
- Takahashi, P. M., Melo, L. P., Frem, R. C. G., Netto, A. V. G., Mauro, A. E., Santos, R. H. A. & Ferreira, J. G. (2006). J. Mol. Struct. 783, 161–167.
- Xing, Y.-H., Han, J., Zhang, B.-L., Zhang, X.-J., Zhang, Y.-H. & Zhou, G.-H. (2006). Acta Cryst. E62, m3354–m3356.
- Zapol’skii, V. & Kaufmann, D. (2008). Technische Universität Clausthal. Unpublished work.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S160053681402460X/wm5084sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681402460X/wm5084Isup2.hkl
CCDC reference: 1033485
Additional supporting information: crystallographic information; 3D view; checkCIF report





