Abstract
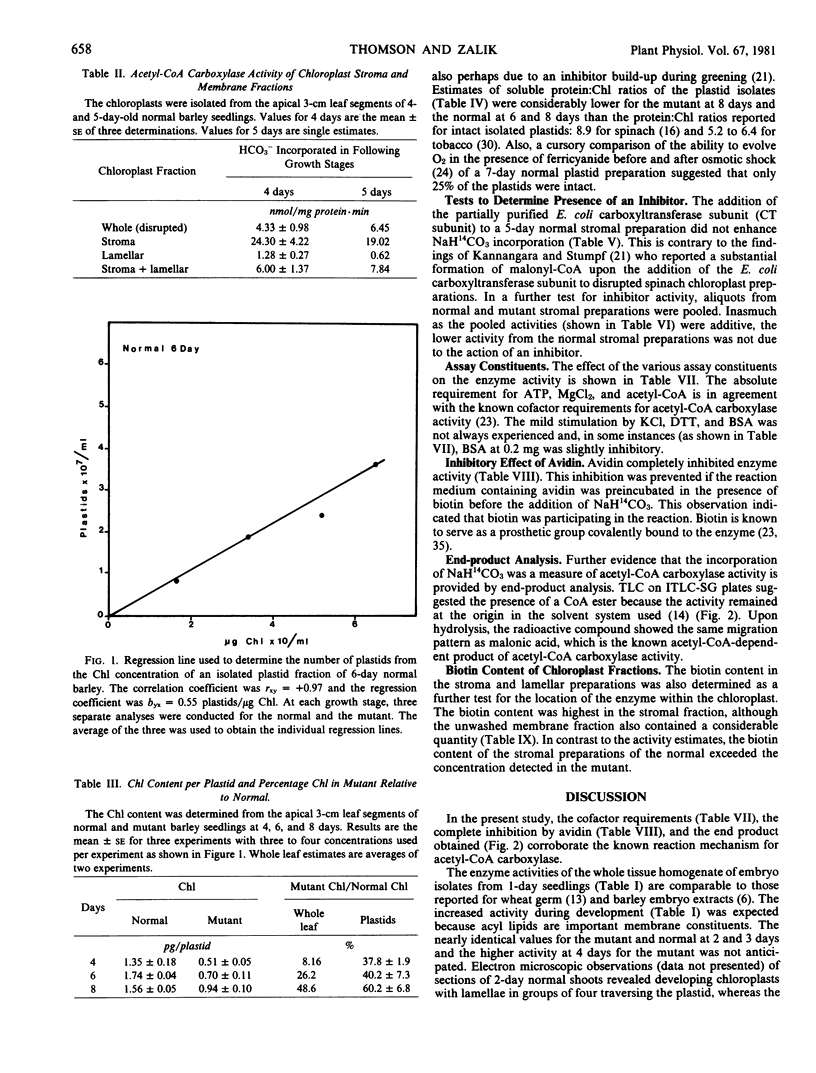
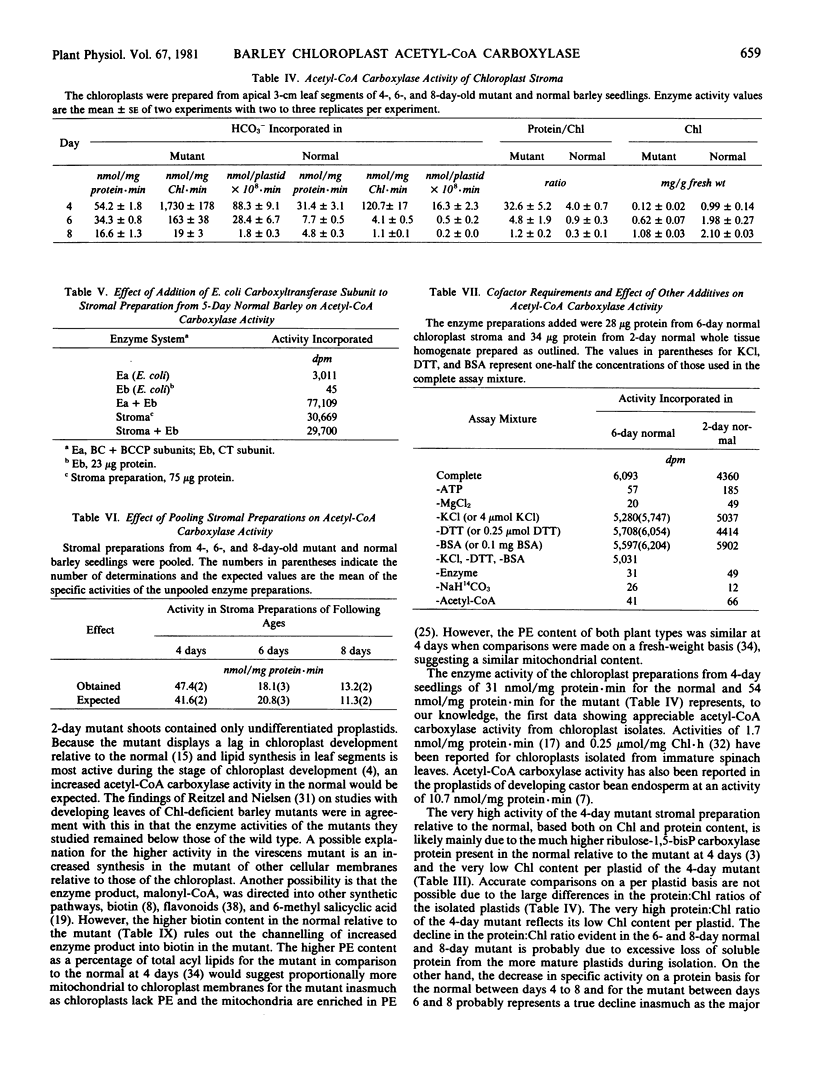
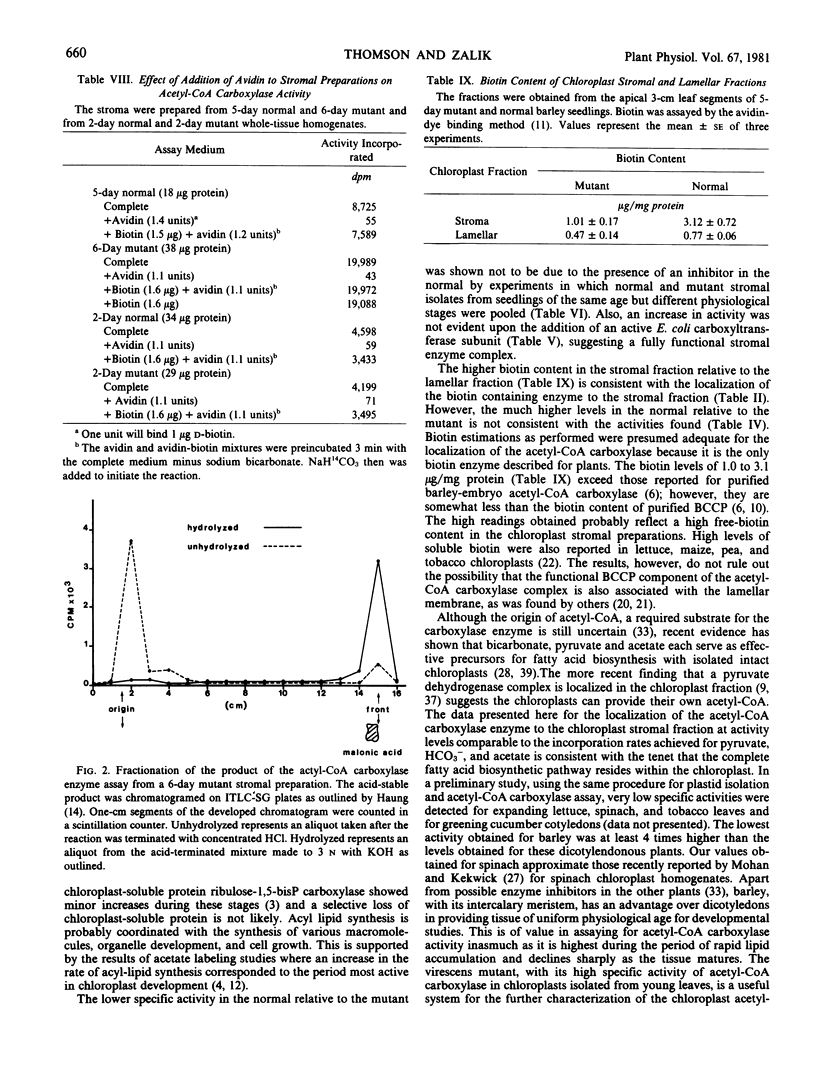
Acetyl coenzyme A (CoA) carboxylase activity of whole tissue homogenates and chloroplast preparations was analyzed as the acetyl-CoA-dependent incorporation of [14C]bicarbonate into an acid-stable product. The absolute requirement for ATP and MgCl2, the complete inhibition with avidin, and end-product analysis were consistent with the presence of acetyl-CoA carboxylase activity. Little difference was found between the mutant and normal tissue homogenates from the 1- to 3-day growth stages, during which period both showed a 3-fold increase. However, by 4 days, the activity of the mutant exceeded that of the normal. Fractionation studies showed that the enzyme was a soluble protein present in the stromal fraction of chloroplasts. The biotin content was also highest in the stroma, although it was found in the lamellar fraction as well. For both the mutant and the normal, the highest acetyl-CoA carboxylase activities were obtained in the stromal preparations from 4-day seedlings (54 and 31 nmoles per milligram protein per minute for the mutant and the normal, respectively) with a progressive decline by 6 and 8 days. The difference between the mutant and the normal was not due to the accumulation of an inhibitor in the normal.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Gordon S. G., Vagelos P. R. Acetyl CoA carboxylase: the purified transcarboxylase component. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1259–1263. doi: 10.1073/pnas.68.6.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barankiewicz T. J., Popovic R. B., Zalik S. Ribulose-1, 5-bisphosphate carboxylase and phosphoenolpyruvate carboxylase activity in barley and its virescens mutant. Biochem Biophys Res Commun. 1979 Apr 13;87(3):884–889. doi: 10.1016/0006-291x(79)92040-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Eisenberg M. A. Biotin: biogenesis, transport, and their regulation. Adv Enzymol Relat Areas Mol Biol. 1973;38:317–372. doi: 10.1002/9780470122839.ch7. [DOI] [PubMed] [Google Scholar]
- Fall R. R., Vagelos P. R. Acetyl coenzyme A carboxylase. Molecular forms and subunit composition of biotin carboxyl carrier protein. J Biol Chem. 1972 Dec 25;247(24):8005–8015. [PubMed] [Google Scholar]
- Heinstein P. F., Stumpf P. K. Fat metabolism in higher plants. 38. Properties of wheat germ acetyl coenzyme A carboxylase. J Biol Chem. 1969 Oct 10;244(19):5374–5381. [PubMed] [Google Scholar]
- Huang K. P. A sensitive assay method of acetyl CoA synthetase. Anal Biochem. 1970 Sep;37(1):98–104. doi: 10.1016/0003-2697(70)90263-0. [DOI] [PubMed] [Google Scholar]
- Joyard J., Douce R. Site of synthesis of phosphatidic acid and diacyglycerol in spinach chloroplasts. Biochim Biophys Acta. 1977 Feb 23;486(2):273–285. doi: 10.1016/0005-2760(77)90023-6. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Henningsen K. W., Stumpf P. K., Appelqvist L. A., von Wettstein D. Lipid biosynthesis by isolated barley chloroplasts in relation to plastid development. Plant Physiol. 1971 Nov;48(5):526–531. doi: 10.1104/pp.48.5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara C. G., Henningsen K. W., Stumpf P. K., von Wettstein D. 6-Methylsalicylic acid synthesis by isolated barley chloroplasts. Eur J Biochem. 1971 Aug 16;21(3):334–338. doi: 10.1111/j.1432-1033.1971.tb01473.x. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Jacobson B. S., Stumpf P. K. Fat Metabolism in Higher Plants: LVII. A Comparison of Fatty Acid-Synthesizing Enzymes in Chloroplasts Isolated from Mature and Immature Leaves of Spinach. Plant Physiol. 1973 Aug;52(2):156–161. doi: 10.1104/pp.52.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara C. G., Jensen C. J. Biotin carboxyl carrier protein in barley chloroplast membranes. Eur J Biochem. 1975 May;54(1):25–30. doi: 10.1111/j.1432-1033.1975.tb04109.x. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Stumpf P. K. Fat metabolism in higher plants. LIV. A procaryotic type acetyl CoA carboxylase in spinach chloroplasts. Arch Biochem Biophys. 1972 Sep;152(1):83–91. doi: 10.1016/0003-9861(72)90196-8. [DOI] [PubMed] [Google Scholar]
- Kannangara C. G., Stumpf P. K. Fat metabolism in higher plants. LVI. Distribution and nature of biotin in chloroplasts of different plant species. Arch Biochem Biophys. 1973 Apr;155(2):391–399. doi: 10.1016/0003-9861(73)90128-8. [DOI] [PubMed] [Google Scholar]
- Miller A. L., Levy H. R. Rat mammary acetyl coenzyme A carboxylase. I. Isolation and characterization. J Biol Chem. 1969 May 10;244(9):2334–2342. [PubMed] [Google Scholar]
- Mohan S. B., Kekwick R. G. Acetyl-coenzyme A carboxylase from avocado (Persea americana) plastids and spinach (Spinacia oleracea) chloroplasts. Biochem J. 1980 Jun 1;187(3):667–676. doi: 10.1042/bj1870667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongun A., Thomson W. W., Mudd J. B. Lipid composition of chloroplasts isolated by aqueous and nonaqueous techniques. J Lipid Res. 1968 Jul;9(4):409–415. [PubMed] [Google Scholar]
- Reitzel L., Nielsen N. C. Acetyl-CoA carboxylase during development of plastids in wild-type and mutant barley seedlings. Eur J Biochem. 1976 May 17;65(1):131–138. doi: 10.1111/j.1432-1033.1976.tb10397.x. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. On the control of long-chain-fatty acid synthesis in isolated intact spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Nov 15;184(2):193–202. doi: 10.1042/bj1840193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson L. W., Zalik S. Acyl lipids, pigments, and gramine in developing leaves of barley and its virescens mutant. Plant Physiol. 1981 Apr;67(4):646–654. doi: 10.1104/pp.67.4.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J. J., Vagelos P. R. Mechanisms and regulation of biosynthesis of saturated fatty acids. Physiol Rev. 1976 Apr;56(2):339–417. doi: 10.1152/physrev.1976.56.2.339. [DOI] [PubMed] [Google Scholar]
- Weaire P. J., Kekwick R. G. The synthesis of fatty acids in avocado mesocarp and cauliflower bud tissue. Biochem J. 1975 Feb;146(2):425–437. doi: 10.1042/bj1460425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M., Randall D. D. Pyruvate Dehydrogenase Complex from Chloroplasts of Pisum sativum L. Plant Physiol. 1979 Dec;64(6):1099–1103. doi: 10.1104/pp.64.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]


