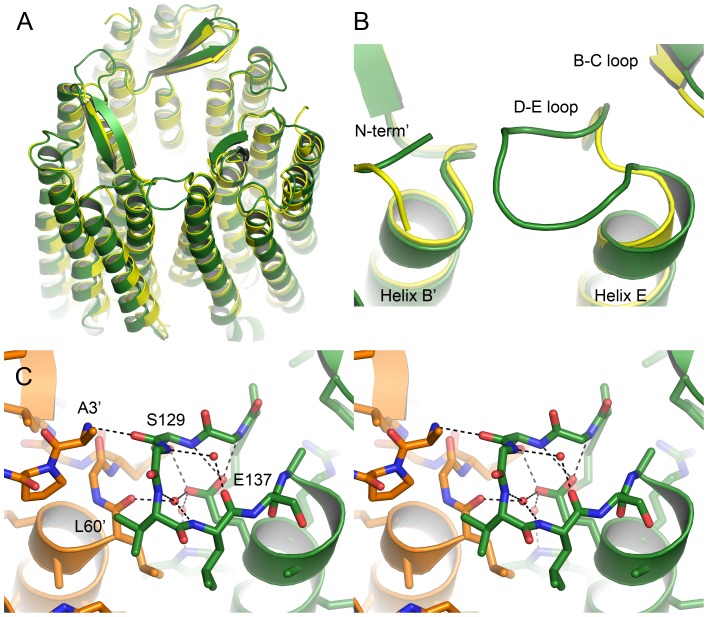
Figure 7. Structure of the HmBRI trimer and its D-E loop.
(A) Comparison of the HmBRI trimer structure (green) with that of HsBR [23] (yellow). HmBRI trimer aligns well in the extracellular region, but the protomers are slightly rotated at the cytoplasmic side. (B) Magnification of the D-E loop. Unlike in other trimerizing retinylidene proteins, in HmBRI the loop is extended and makes contact to the adjacent protomer. (C) Wall-eyed stereogram of the HmBRI D-E loop. The adjacent protomer is shown in orange and its residues are marked by a prime. Three structural water molecules are observed that stabilize the loop structure.