Figure 1. Down-regulation of eEF1A reduces the amount of infectious virus released from RSV-infected cells.
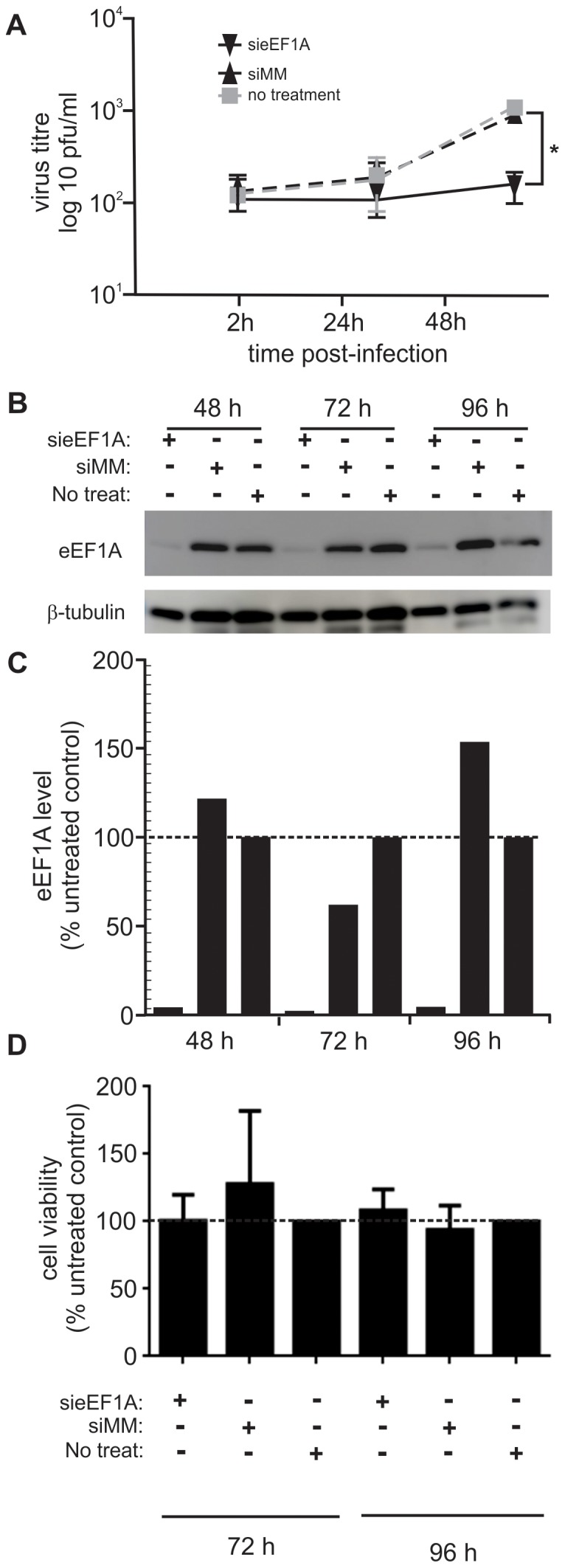
HEK293T cells were transfected with sieEF1A, or a non-specific control siRNA (siMM), 48 h prior to infection with RSV A2 at a MOI of 0.1 pfu/cell. Infectious virus released from the cells into the culture supernatant was quantified by immune-plaque assay using Hep-2a cells exposed to culture supernatants, then overlayed with methyl cellulose and incubated at 37°C for 6 days. RSV-positive plaques were detected using antisera to RSV. (A) The amount of infectious virus released by cells in which eEF1A had been down-regulated was significantly reduced 48 h post-infection, compared to cells that had been transfected with the siMM control or untreated. (B) Down-regulation of eEF1A>90% by sieEF1A and not the siMM control was confirmed by western blot analysis of cell lysates at the time of infection, which was 48 h after transfection and also for the 24 h and 48 h following RSV infection (72 h and 96 h post-transfection). Beta-Tubulin was used as a loading control. (C) A digitized western blot in (B) was analyzed using ImageJ software. The eEF1A level (average pixel intensity) in each lane was normalized to the corresponding level of β-tubulin in the same lane. For each time point, the amount of eEF1A detected in untreated control samples was designated as 100%. (D) HEK293T cells transfected with either sieEF1A or siMM, remained viable compared to untreated cells 72 h and 96 h post-transfection. The average cell viability compared with an uninfected control was calculated. siRNA experiments were repeated three times with similar results. Mean values, with SEM are shown. Significance identified using paired t-test. *P<0.05
