Abstract
Decabromodiphenyl ether (decaBDE) adversely affects reproduction and development. Our previous study showed that postnatal exposure to a low dose of decaBDE (0.025 mg/kg body weight/day) by subcutaneous injection on postnatal days (PNDs) 1 through 5 leads to reductions in testicular size and number of Sertoli cells and sperm, while higher dose of decaBDE (2.5 mg/kg body weight/day) had no significant differences about these. In the present study, we examined the molecular mechanism of these effects on mouse testes following postnatal exposure to a low decaBDE dose. We hypothesized that postnatal exposure to decaBDE may alter levels of serum thyroid hormones (THs) and testosterone, or the level of TH receptor alpha (Thra) transcripts and its splicing variants and androgen receptor (Ar) in Sertoli cells, adversely affecting spermatogenesis. To test this hypothesis, we examined serum TH and testosterone levels and the levels of transcripts of the Ar, Thra and its splicing variants, and Thra splicing factors (Hnrnpa1, Srsf1, and Hnrnph1) with qPCR in isolated mouse Sertoli cells exposed postnatally to decaBDE (0.025, 0.25, and 2.5 mg/kg). Levels of serum testosterone and transcripts encoding Ar, Thra, and its variant, Thra1, declined significantly in Sertoli cells of mice exposed to 0.025 mg decaBDE/kg. No significant differences in serum TH level or Thra2, Hnrnph1, or Srsf1 transcript levels were observed between control and decaBDE-exposed mice. However, the Thra1:Thra2 and Hnrnpa1:Srsf1 ratios were altered in Sertoli cells of mice exposed to 0.025 mg decaBDE/kg but not in cells exposed to 0.25 or 2.5 mg decaBDE/kg. These results indicate that postnatal exposure to a low dose of decaBDE on PNDs 1 through 5 lowers the testosterone level and the levels of Ar and Thra transcripts in Sertoli cells, accompanied by an imbalance in the ratios of Thra splicing variants, resulting in smaller testicular size and impaired spermatogenesis.
Introduction
The polybrominated diphenyl ether (PBDE), decabromodiphenyl ether (decaBDE), is widely used as a flame retardant and is a common environmental pollutant. PBDEs have thyroid-disrupting effects due to structural similarities with thyroid hormones (THs) [1]–[3]. DecaBDE also reportedly disrupts TH levels in fish and rodents [4]–[7]. Similar to other studies [8]–[10], we previously showed that decaBDE exhibits male reproductive toxicity in mice. Postnatal exposure of mice to a low dose (0.025 mg/kg body weight) of decaBDE leads to reduced testicular weight, lower numbers of Sertoli cells and elongated spermatids, and reduced sperm count [11]. However, the detailed mechanism underlying these effects remains unclear.
Sertoli cells play a critical role in spermatogenesis by supporting germ cell differentiation in testicular seminiferous tubules through a number of hormones [12]. Several THs (thyroxine [T4] and triiodothyronine [T3], but especially T3) play important roles in regulating Sertoli cell proliferation and maturation by binding to TH receptors (TRs) [13], [14]. Two isoforms in mice have been identified, TRα and TRβ, which are encoded by the genes Thra and Thrb, respectively [15], [16]. Thra has three splicing variants (Thra1, Thra2, and Thra3), whereas Thrb has two splicing variants (Thrb1 and Thrb2) [17], [18]. Although Sertoli cells express the TRα isozymes encoded by Thra1, Thra2, and Thra3 and the TRβ isozyme encoded by Thrb1, binding of the isozyme encoded by Thra1 to T3 is necessary for normal maturation of Sertoli cells [13]. The androgen receptor (AR) is also expressed in Sertoli cells [19], [20]. In Sertoli cells, the AR functions in the maintenance of testicular development, thereby helping to guarantee male fertility [21], [22]. Similar to testosterone, exposure to T3 reportedly increases the level of Ar transcripts in cultured Sertoli cells [23].
Serine/arginine-rich splicing factor 1 (SRSF1) plays an important role in regulating alternative splicing by associating with heterogeneous nuclear ribonucleoprotein A1 (HNRNPA1) [24], [25]. SRSF1 and HNRNPA1 have opposing actions in vivo [26], with HNRNPA1 functioning as a repressor [27]. Thus, alterations in the SRSF1 to HNRNPA1 ratio affect the alternative splicing of genes [25]. SRSF1 and heterogeneous nuclear ribonucleoprotein H1 (HNRNPH1) reportedly regulate the splicing of Thra2 by binding to purine residues within a Thra2-specific 5′ splicing site [28]. Thus, there appears to be an inverse correlation between the Hnrnpa1:Srsf1 and Thra1:Thra2 expression ratios [28].
Based on the above data, we hypothesized that the molecular mechanism underlying the effects resulting from exposure of mice to low doses of decaBDE on postnatal days (PNDs) 1 through 5 involves changes in the normal levels of serum THs and testosterone or transcripts of Ar, Thra and its splicing variants in Sertoli cells, resulting in the disruption of spermatogenesis. To test this hypothesis, we examined serum TH and testosterone levels and the levels of transcripts encoding Ar, Thra and its splicing variants, and the levels of transcripts encoding the Thra2 splicing factors Hnrnpa1, Srsf1, and Hnrnph1 in Sertoli cells isolated from testes of mice exposed postnatally to decaBDE.
Materials and Methods
Ethics Statement
All experimental procedures were performed in accordance with the Chiba University Guide for the Care and Use of Laboratory Animals. The study and protocol were approved by the Ethics Committee of Chiba University, Graduate School of Medicine (Approval numbers: 26–91 and 25–141). Mice were sacrificed at 12 weeks of age (PND 84) by CO2 euthanasia, after which whole blood and testes were collected.
Animals
Twelve-week-old ICR male (n = 12) and female (n = 12) mice were purchased from Japan SRL (Hamamatsu, Japan) and then mated. The pregnant dams were randomly divided into four groups. After birth, five male pups were selected from each litter. The mice were housed in a temperature-controlled room (24–26°C) under a 12-h light/dark cycle. CLEA rodent chow CE2 (CLEA Japan Inc., Tokyo, Japan) and water were provided ad libitum.
Reagents
DecaBDE was purchased from Wako Pure Chemical Industries (Osaka, Japan). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise indicated.
Administration of decaBDE
Mice were administered decaBDE at doses of 0.025, 0.25, or 2.5 mg/kg body weight/day by subcutaneous injection on PNDs 1 through 5, as previously described [11]. Control group mice were injected similarly with corn oil only.
Measurement of serum TH (T3, T4, free T3, and free T4) and testosterone levels
Blood samples were collected by cardiac puncture from control and decaBDE-exposed mice. Serum was prepared by centrifugation of whole blood at 880 g for 20 min at room temperature. The levels of serum T3 and T4 were measured using rodent triiodothyronine and thyroxine ELISA test kits (Endocrine Technologies Inc., Newark, CA, USA), respectively, according to the manufacturer's instructions. Similarly, the levels of free T3 (FT3) and free T4 (FT4) in the serum were measured using free tri-iodothyronine indes and free thyroxine ELISA kits (Cusabio Biotech Co., Wuhan, PR China), respectively, according to the manufacturer's instructions. A rodent testosterone ELISA kit (Endocrine Technologies) was used to measure serum testosterone levels.
Establishment of primary Sertoli cell culture
Testes from control and decaBDE-exposed mice were resected and the tunica albuginea was removed. The seminiferous tubules were incubated at room temperature for 2 h with gentle agitation in Eagle's minimal essential medium (pH 7.2) (Sigma-Aldrich) containing 0.1% collagenase (Wako Pure Chemical Industries). The suspensions of the control and the decaBDE-exposed mice were removed from agitation and allowed to stand for 5 min to precipitate the seminiferous tubules. The supernatants were discarded and the seminiferous tubules were dispersed gently in PBS containing 1 mM EDTA and placed at room temperature for two-three minutes. The suspensions were centrifuged at 600× g for 10 min, and the resulting supernatants were discarded. The tubules were then incubated with 0.25% trypsin (Sigma-Aldrich) for 10 min, after which each suspension was filtered through a nylon mesh (40 µm, Corning Inc., Corning, NY, USA) and centrifuged at 600× g for 10 min. The supernatant was discarded and the tubules were washed once with PBS. The tubules were dispersed in Eagle's minimal essential medium (pH 7.2) containing 10% FBS (Gibco, Carlsbad, CA, USA) and 1% penicillin-streptomycin (Sigma-Aldrich), plated on collagen-coated culture dishes (Iwaki, Tokyo, Japan), and incubated at 32.5°C in a 5% CO2 atmosphere to allow for growth of Sertoli cells. After 2 h, the medium was replaced and the cells were continuously incubated at 32.5°C in a 5% CO2 atmosphere. After 1 week, the Sertoli cells were collected and stored at −80°C until used.
RNA extraction and real-time PCR (qPCR)
Total RNA was extracted from isolated Sertoli cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Reverse-transcription of RNA for real-time PCR was performed with a QuantiTect Reverse Transcription kit (Qiagen, Valencia, CA, USA), according to the manufacturer's protocol. A DNA Engine Opticon (MJ Research Inc, Cambridge, MA, USA) and SYBR Green Real-time PCR Master Mix (Toyobo, Osaka, Japan) were used for real-time PCR, according to the manufacturers' instructions. The primer sequences used for qPCR are shown in Table 1. Briefly, the thermocycling program consisted of one cycle at 95°C for 1 min, 40 cycles of denaturation at 95°C for 15 s, and annealing plus elongation for 1 min at 49.9°C for Thra, 68.2°C for Thra1, 66.5°C for Thra2, 68.2°C for Ar, 68.2°C for Hnrnpa1, 68.2°C for Srsf1, 54.0°C for Hnrnph1, or 68.2°C for beta-actin (Actb). The level of each mRNA transcript was normalized to that of Actb as an internal control.
Table 1. Primer pairs used for qPCR.
| Gene | Primer sequences | Primer position | Amplified size (bp) | GenBank accession number |
| Thra | For: 5′-GGA TGG AAT TGA AGT GAA TGG AA-3′ | 414–436 | 109 | NM_178060.3 |
| Rev: 5′-CCG TTC TTT CTT TTT CGC TTT C-3′ | 500–522 | |||
| Thra1 | For: 5′- CTG CCT TGC GAA GAC CAG ATC-3′ | 865–884 | 307 | X07750.1 |
| Rev: 5′-CGA CTT TCA TGT GGA GGA AG-3′ | 1,152–1,171 (Thra1specific) | |||
| Thra2 | For: 5′-AAT GGT GGC TTG GGT GTG GT-3′ | 865–884 | 364 | X07751.1 |
| Rev: 5′-CCT GAA CAA CAT GCA TTC CGA-3′ | 1,208–1,228 (Thra2 specific) | |||
| Ar | For: 5′-GCC CCC ATC CAA GAC CTA TCG-3′ | 71–91 | 145 | NM_013476.3 |
| Rev: 5′-GCT AGT CTC CTG CCT CTG CTG-3′ | 195–215 | |||
| Hnrnpa1 | For: 5′-TGG AAG CAA TTT TGG AGG TGG-3′ | 946–966 | 155 | BC092395.1 |
| Rev: 5′-GGT TCC GTG GTT TAG CAA AGT-3′ | 1,080–1,100 | |||
| Srsf1 | For: 5′-CAC TGG TGT CGT GGA GTT TG-3′ | 566–585 | 189 | BC046773 |
| Rev: 5′-CTT CTG CTA CGG CTT CTG CT-3′ | 735–754 | |||
| Hnrnph1 | For: 5′-ATT GCA TAG GTA GCC AAG G-3′ | 1,396–1,414 | 119 | BC056224.1 |
| Rev: 5′-CCA TCC ACC ACT CAT ACT AGA C-3′ | 1,493–1,514 | |||
| Hnrnpf | For: 5′-GGC ATC TGT GGT GGT TCT TT-3′ | 226–245 | 175 | NM_133834.2 |
| Rev: 5′-TGC AGT CGG AGA GGA AGT TT-3′ | 381–400 | |||
| Actb | For: 5′-AGA GGG AAA TCG TGC GTG AC-3′ | 693–712 | 138 | NM_007393.3 |
| Rev: 5′-CAA TAG TGA TGA CCT GGC CGT-3′ | 810–830 |
Statistical analysis
Results were evaluated for statistical significance using one-way ANOVA followed by Dunnett's test. All values are expressed as the mean and standard deviation (SD). A P value <0.05 was considered to indicate a significant difference.
Results
No significant changes were detected in serum TH levels between control and decaBDE-exposed groups
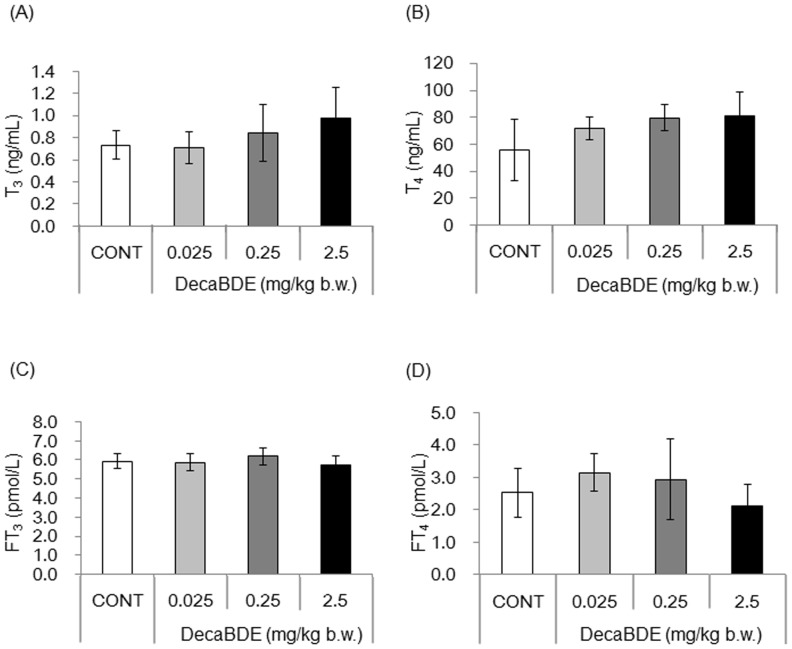
To determine whether postnatal exposure to decaBDE disrupts normal TH levels, we measured the concentrations of serum T3, T4, FT3, and FT4 by ELISA. Although the serum levels of T3 and T4 tended to increase in a dose-dependent manner, no significant difference was observed between the control and decaBDE-exposed mice (Fig. 1A, B). In addition, no significant changes in the levels of serum FT3 and FT4 were observed between the control and decaBDE-exposed mice (Fig. 1C, D).
Figure 1. Serum TH levels in control and decaBDE-exposed mice.
Total T3 (A), total T4 (B), FT3 (C) and FT4 (D) levels in the serum of control and decaBDE-exposed mice were measured by ELISA. Each value is the mean ± SD of 6 samples per group.
Serum testosterone levels were decreased in the 0.025 and 0.25 mg/kg exposed-groups
The effect of postnatal exposure to decaBDE on serum testosterone levels was evaluated by ELISA. Serum testosterone levels were significantly lower in mice exposed to decaBDE at 0.025 and 0.25 mg/kg than in the controls (Fig. 2). There was no significant difference in testosterone level between the control mice and mice exposed to decaBDE at 2.5 mg/kg.
Figure 2. Serum testosterone levels in control and decaBDE-exposed mice.
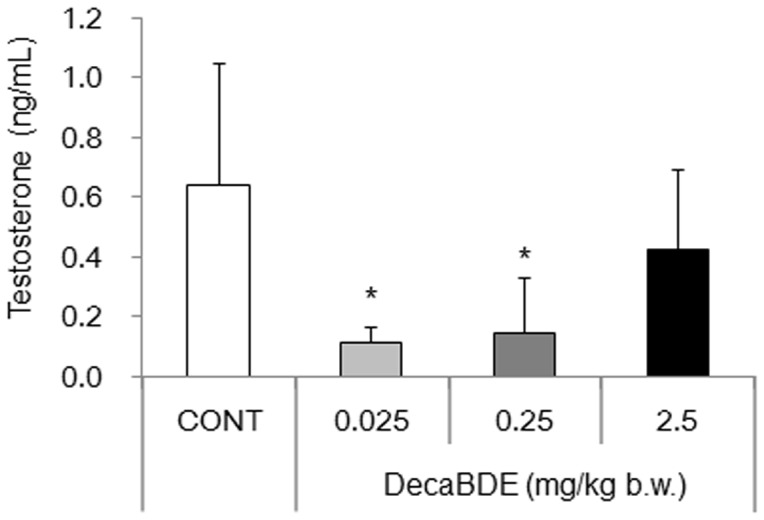
Testosterone levels in the serum of control and decaBDE-exposed mice were measured by ELISA. Each value is the mean ± SD of 6 samples per group. *P<0.05 compared with the control.
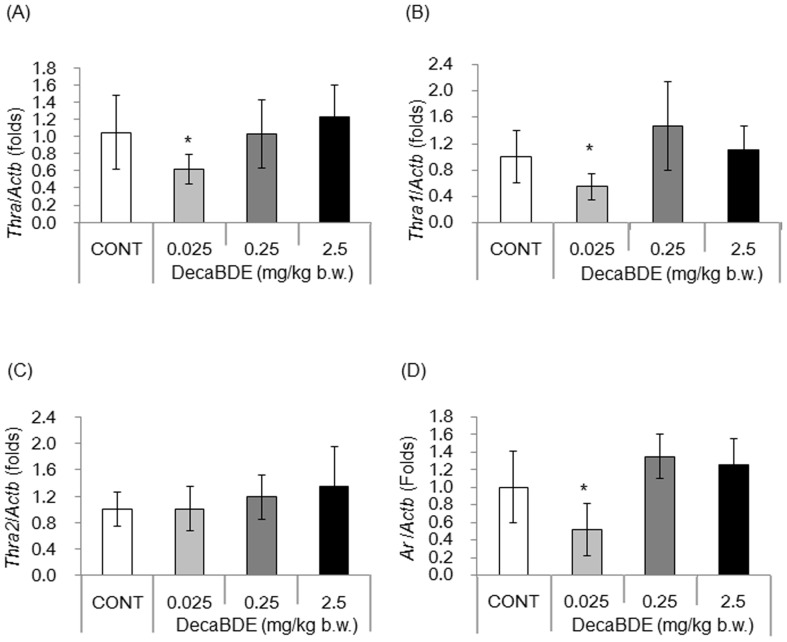
Expression levels of Thra and its splicing variant (Thra1) were reduced in isolated Sertoli cells of mice exposed to 0.025 mg decaBDE/kg
We confirmed that THRA is expressed in Sertoli cells (Fig. S1). Other studies have shown that transcripts of Thra1 and Thra2 are also expressed in Sertoli cells [29]. The effect of postnatal exposure to decaBDE on expression of the Thra gene and its splicing variants (Thra1 and Thra2) in Sertoli cells was evaluated by determining the levels of respective transcripts using qPCR with cDNAs obtained from isolated Sertoli cells. The level of Thra transcripts was significantly lower in mice exposed to decaBDE at 0.025 mg/kg than in controls (Fig. 3A), whereas no significant differences were observed at higher decaBDE doses. The level of Thra1 transcripts was significantly lower in mice exposed to decaBDE at 0.025 mg/kg, whereas no significant differences were observed at higher decaBDE doses (Fig. 3B). The level of Thra2 transcripts was slightly higher in decaBDE-exposed mice compared with controls; however, the differences were not significant (Fig. 3C).
Figure 3. Levels of Thra and Ar transcripts in isolated Sertoli cells.
Levels of transcripts of Thra (A), Thra1 (B), Thra2 (C), and Ar (D) were measured using qPCR in control and decaBDE-exposed isolated Sertoli cells cultured for 1 week. Transcript expression was normalized to the level of Actb transcript expression and is shown as the ratio relative to Actb compared with the control (set as a value of 1.0). Each value is the mean ± SD of 6-9 samples per group. *P<0.05 compared with the control.
Transcript levels of Ar were decreased in isolated Sertoli cells of mice exposed to 0.025 mg decaBDE/kg
As T3 reportedly upregulates transcription of Ar in Sertoli cells in vitro [23], we measured the level of Ar transcripts to assess the effect of decaBDE exposure on expression of this gene. Interestingly, the level of Ar transcripts in isolated Sertoli cells exposed to decaBDE at 0.025 mg/kg was significantly lower than in controls (Fig. 3D). Although the level of Ar transcripts increased slightly in mice exposed to decaBDE at 0.25 and 2.5 mg/kg, the differences were not significant between the control and the dose groups.
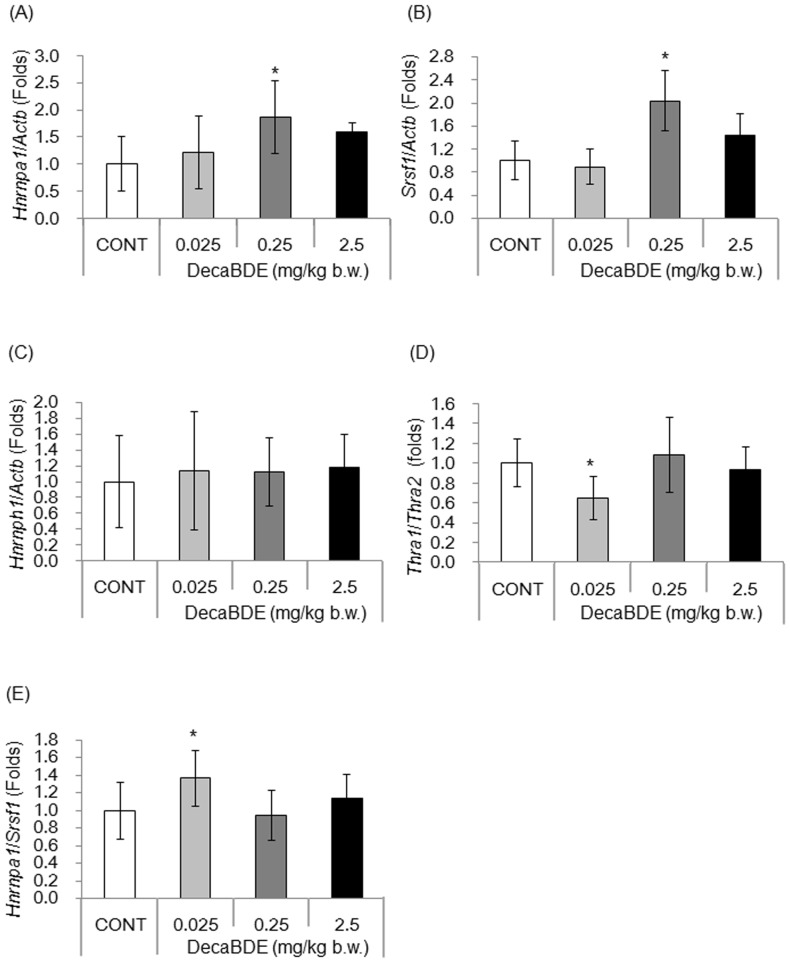
Expression levels of Hnrnpa1 and Srsf1 were increased in isolated Sertoli cells of mice exposed to 0.25 mg decaBDE/kg
We confirmed that the Thra2 splicing factors HNRNPA1, SRSF1, and HNRNPH1 were expressed in Sertoli cells (Fig. S2) and thus were available to mediate splicing of the Thra gene. The effect of postnatal exposure to decaBDE on regulation of Thra splicing in isolated Sertoli cells was evaluated by measuring the levels of Hnrnpa1and Srsf1 transcripts. The levels of Hnrnpa1 and Srsf1 transcripts were significantly higher in mice exposed to decaBDE at 0.25 mg/kg than in the controls (Fig. 4A, B). No significant differences in the levels of Hnrnpa1and Srsf1 transcripts were observed at lower and higher decaBDE dose groups (Fig. 4A, B). Exposure to decaBDE had no effect on Hnrnph1 expression between the control and dose groups (Fig. 4C).
Figure 4. Levels of splicing factor transcripts in isolated Sertoli cells.
Levels of transcripts of Hnrnpa1 (A), Srsf1 (B), and Hnrnph1 (C), the Thra1:Thra2 transcript expression ratio (D), and the Hnrnpa1:Srsf1 transcript expression ratio (E) were measured using qPCR in isolated Sertoli cells cultured for 1 week. Each value is normalized to the transcript level of Actb and is shown as the ratio relative to the control (set as a value of 1.0). Each value is the mean ± SD of 6–9 samples per group. *P<0.05 compared with the control.
Expression ratios of Thra1:Thra2 and Hnrnpa1:Srsf1 were oppositely altered in isolated Sertoli cells of mice exposed to 0.025 mg decaBDE/kg
The Thra1:Thra2 expression ratio was significantly lower in Sertoli cells of mice exposed to decaBDE at 0.025 mg/kg than in controls (Fig. 4D), whereas no significant differences were observed at higher decaBDE dose groups (Fig. 4D).
The Hnrnpa1:Srsf1 expression ratio was significantly higher in Sertoli cells of mice exposed to 0.025 mg decaBDE/kg than in controls (Fig. 4E), whereas no significant differences were observed at higher decaBDE dose groups. Thus, exposure to a low (0.025 mg/kg) dose of decaBDE had opposite effects on the Thra1:Thra2 and Hnrnpa1:Srsf1 expression ratios in isolated Sertoli cells.
Discussion
Our previous study reported that early postnatal exposure to a low dose (0.025 mg/kg) of decaBDE has adverse effects in male mice: reduced testicular weight, lower numbers of Sertoli cells and elongated spermatids, and reduced sperm count [11]. However, these changes were not observed in the high dose (2.5 mg/kg) decaBDE-exposed group. These findings suggest that different mechanism may be involved following postnatal exposure to low and high doses of decaBDE.
Although we hypothesized that postnatal exposure to decaBDE may disrupt normal serum TH levels due to similarities in chemical structure [1]–[3], we found that postnatal exposure to decaBDE from PND 1 through 5 had no impact on serum TH levels (Fig. 1). In mice, development of the thyroid glands begins 8.5–10 days postconception appears 17.5 days postconception [30], [31]. The period of decaBDE administration in this study did not encompass the window of thyroid gland development, which could explain why we observed no effect on serum TH levels.
In contrast to serum THs, exposure to decaBDE at 0.025 or 0.25 mg/kg resulted in a significant reduction in serum testosterone levels (Fig. 2). This finding is consistent with that of another study [9] involving a different decaBDE dose. In addition, the level of Ar transcripts in isolated Sertoli cells declined significantly following exposure to decaBDE 0.025 mg/kg (Fig. 3E). Testosterone is important for normal spermatogenesis and development of male reproductive organs, acting on target cells via the AR [32], [33]. The phenotype of Sertoli cell-specific Ar gene-deletion mice includes smaller testicular size and no other male reproductive organs (seminal vesicles, prostate, or epididymis) [21]. A previous study showed reduced testicular weight and reduced numbers of Sertoli cells, elongated spermatids, and sperm in mice exposed postnatally to decaBDE at 0.025 mg/kg but no such effects in mice exposed to decaBDE at 0.25 or 2.5 mg/kg [11]. Although we did not examine steroidogenesis or Leydig cell function in the present study, the observed decreases in the levels of serum testosterone and Ar transcripts in Sertoli cells could cause reductions in testicular size and number of sperm in mice dosed postnatally with decaBDE at 0.025 mg/kg. Further studies are necessary to elucidate the mechanism underlying the observed reductions in testosterone and Ar transcript levels following postnatal exposure to decaBDE at 0.025 mg/kg.
We found that the levels of Thra and Thra1 transcripts in isolated Sertoli cells (Fig. 3A, B) decreased significantly following exposure to decaBDE at 0.025 mg/kg. However, the exposure to decaBDE at 0.025 mg/kg had no significant effect on the levels of Thra2, Hnrnph1, or Srsf1 transcripts in isolated Sertoli cells (Fig. 3C and Fig. 4B, C). These findings suggest that postnatal exposure to decaBDE on PNDs 1 through 5 has no impact on Thra2 splicing. At present, we cannot explain the mechanism underlying the down-regulation of Thra and Thra1 expression induced by exposure to the lower dose of decaBDE. It is possible that the Thra1 and Thra2 splicing processes differ. In mice, Sertoli cells develop in the fetal period and end by PND 15 [34], [35]. As the administration period of this study encompassed PNDs 1 through 5, the decaBDE likely affected the development of Sertoli cells, including the splicing process of Thra variants. Moreover, both THRA and AR are members of the nuclear receptor superfamily and are known to be expressed in Sertoli cells [36]. The present study found that expression of the Thra and Ar genes decreased significantly following exposure to decaBDE at 0.025 mg/kg. Although some studies have examined the effect of THs on expression of Thra and Ar genes [23], [37], there are no studies have examined the functional relationship between THRA and AR in Sertoli cells. Further research is needed to elucidate the detail mechanism following early postnatal exposure to decaBDE at 0.025 mg/kg on down-regulation of Thra and Ar genes in Sertoli cells.
Exposure to decaBDE at 0.025 mg/kg disturbed the normal Thra1:Thra2 and Hnrnpa1:Srsf1 expression ratios in isolated Sertoli cells, whereas exposure to higher doses had no significant effect (Fig. 4D, E). Moreover, exposure to the low dose of decaBDE had opposite effects on the Thra1:Thra2 and Hnrnpa1:Srsf1 expression ratios in isolated Sertoli cells, consistent with the results of other studies [28], [38]. The Thra1:Thra2 expression ratio varies based on the tissue or cell type and is affected by changes in physiological conditions, such as may result from fasting [38]–[40]. Our study is the first to demonstrate that chemical exposure may also cause imbalances in the normal Thra1:Thra2 and Hnrnpa1:Srsf1 expression ratios, suggesting that these ratios may be useful as markers of drug-associated toxicity.
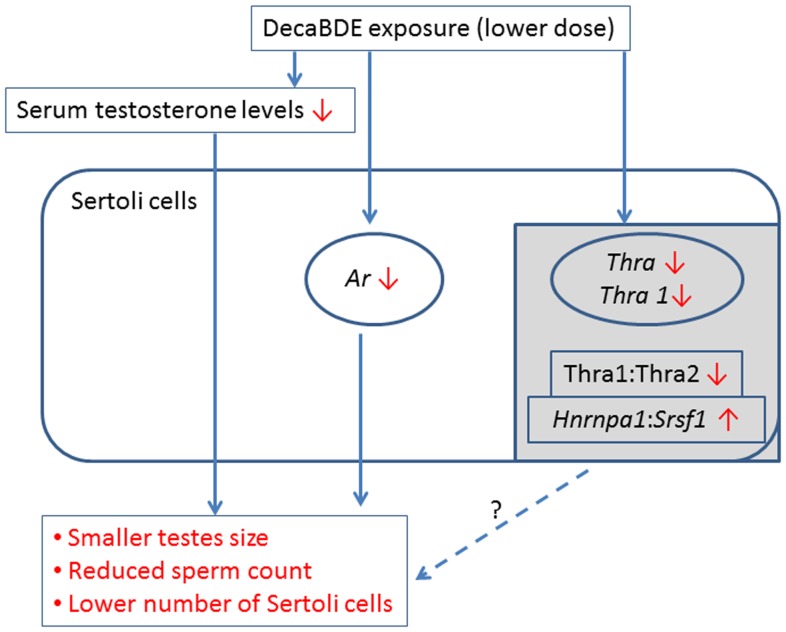
In conclusion, we found that postnatal exposure to a low concentration (0.025 mg/kg) of decaBDE results in significant reductions in the levels of serum testosterone and transcripts of Ar, Thra, and its variant, Thra1 in isolated Sertoli cells (Fig. 5). The same dose leads to significant alterations in the normal Thra1:Thra2 and Hnrnpa1:Srsf1 expression ratios, whereas higher decaBDE doses of 0.25 and 2.5 mg/kg have no significant effect on these ratios. Our results suggest that the above-mentioned changes resulting from postnatal exposure to a low dose of decaBDE on PNDs 1 through 5 are sufficient to cause reduced testicular size and reduced numbers of Sertoli cells and sperm. Further research is necessary to more fully elucidate the detailed mechanism(s) of the toxicity associated with exposure to low decaBDE doses between PND 1 and 5 in both Ledydig and Sertoli cells.
Figure 5. Possible mechanism following early postnatal exposure to a low dose of decaBDE (0.025 mg/kg) in mouse testis.
Early postnatal exposure to decaBDE from PND 1 to 5 causes to reduce serum testosterone level and Ar transcript levels in Sertoli cells, resulting in smaller testes size, reduced sperm count and a lower number of Sertoli cells, as well as decreased transcript levels of Thra and its splicing variant, Thra1, and an imbalance in the expression ratios of Thra1:Thra2 and Hnrnpa1:Srsf1 (gray box). It remains unknown if these latter changes are related to smaller testes size, reduced sperm count and lower number of Sertoli cells (dotted line).
Supporting Information
Expression and localization of THRA in mouse testes. Sections of adult mouse testes were immunostained with antibodies to THRA (A) and the Sertoli cell marker GATA4 (B). Insets show higher magnification. Yellow indicates colocalization of THRA and GATA4 (C). Bars = 50 µm.
(TIF)
Expression and localization of HNRNPA1, SRSF1, and HNRNPH1 in mouse testes. Sections of adult mouse testes were double-immunostained with antibodies to HNRNPA1 (A), SRSF1 (D), HNRNPH (G) and the Sertoli cell marker GATA4 (middle panels, B, E, H). Insets show higher magnification. Yellow indicates colocalization of HNRNPA1, SRSF1, or HNRNPH and GATA4 (C, F, I). Bars = 50 µm.
(TIF)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the JSPS KAKENHI (grant numbers 20241016 and 24710069). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Boas M, Feldt-Rasmussen U, Main KM (2012) Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol 355:240–248. [DOI] [PubMed] [Google Scholar]
- 2. McDonald TA (2002) A perspective on the potential health risks of PBDEs. Chemosphere 46:745–755. [DOI] [PubMed] [Google Scholar]
- 3. Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, et al. (2000) Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci 56:95–104. [DOI] [PubMed] [Google Scholar]
- 4. Lee E, Kim TH, Choi JS, Nabanata P, Kim NY, et al. (2010) Evaluation of liver and thyroid toxicity in Sprague-Dawley rats after exposure to polybrominated diphenyl ether BDE-209. J Toxicol Sci 35:535–545. [DOI] [PubMed] [Google Scholar]
- 5. Noyes PD, Lema SC, Macaulay LJ, Douglas NK, Stapleton HM (2013) Low level exposure to the flame retardant BDE-209 reduces thyroid hormone levels and disrupts thyroid signaling in fathead minnows. Environmental science & technology 47:10012–10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim TH, Lee YJ, Lee E, Kim MS, Kwack SJ, et al. (2009) Effects of gestational exposure to decabromodiphenyl ether on reproductive parameters, thyroid hormone levels, and neuronal development in Sprague-Dawley rats offspring. J Toxicol Environ Health A 72:1296–1303. [DOI] [PubMed] [Google Scholar]
- 7. Tseng LH, Li MH, Tsai SS, Lee CW, Pan MH, et al. (2008) Developmental exposure to decabromodiphenyl ether (PBDE 209): effects on thyroid hormone and hepatic enzyme activity in male mouse offspring. Chemosphere 70:640–647. [DOI] [PubMed] [Google Scholar]
- 8. Tseng LH, Lee CW, Pan MH, Tsai SS, Li MH, et al. (2006) Postnatal exposure of the male mouse to 2,2′,3,3′,4,4′,5,5′,6,6′-decabrominated diphenyl ether: decreased epididymal sperm functions without alterations in DNA content and histology in testis. Toxicology 224:33–43. [DOI] [PubMed] [Google Scholar]
- 9. Tseng LH, Hsu PC, Lee CW, Tsai SS, Pan MH, et al. (2013) Developmental exposure to decabrominated diphenyl ether (BDE-209): effects on sperm oxidative stress and chromatin DNA damage in mouse offspring. Environmental toxicology 28:380–389. [DOI] [PubMed] [Google Scholar]
- 10. Zhai JX, Wang XH, Zhang ZX, Zou LW, Ding SS (2011) [Studying the lipid peroxidation index, morphology and apoptosis in testis of male BALB/c mice exposed to polybrominated diphenyl ether (BDE-209)]. Zhonghua lao dong wei sheng zhi ye bing za zhi = Zhonghua laodong weisheng zhiyebing zazhi = Chinese journal of industrial hygiene and occupational diseases 29:294–298. [PubMed] [Google Scholar]
- 11. Miyaso H, Nakamura N, Matsuno Y, Kawashiro Y, Komiyama M, et al. (2012) Postnatal exposure to low-dose decabromodiphenyl ether adversely affects mouse testes by increasing thyrosine phosphorylation level of cortactin. J Toxicol Sci 37:987–999. [DOI] [PubMed] [Google Scholar]
- 12. Griswold MD (1998) The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol 9:411–416. [DOI] [PubMed] [Google Scholar]
- 13. Holsberger DR, Cooke PS (2005) Understanding the role of thyroid hormone in Sertoli cell development: a mechanistic hypothesis. Cell Tissue Res 322:133–140. [DOI] [PubMed] [Google Scholar]
- 14. Mullur R, Liu YY, Brent GA (2014) Thyroid hormone regulation of metabolism. Physiol Rev 94:355–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sap J, Munoz A, Damm K, Goldberg Y, Ghysdael J, et al. (1986) The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature 324:635–640. [DOI] [PubMed] [Google Scholar]
- 16. Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, et al. (1986) The c-erb-A gene encodes a thyroid hormone receptor. Nature 324:641–646. [DOI] [PubMed] [Google Scholar]
- 17. O′Shea PJ, Williams GR (2002) Insight into the physiological actions of thyroid hormone receptors from genetically modified mice. J Endocrinol 175:553–570. [DOI] [PubMed] [Google Scholar]
- 18. Cheng SY, Leonard JL, Davis PJ (2010) Molecular aspects of thyroid hormone actions. Endocrine reviews 31:139–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shan LX, Zhu LJ, Bardin CW, Hardy MP (1995) Quantitative analysis of androgen receptor messenger ribonucleic acid in developing Leydig cells and Sertoli cells by in situ hybridization. Endocrinology 136:3856–3862. [DOI] [PubMed] [Google Scholar]
- 20. Shan LX, Bardin CW, Hardy MP (1997) Immunohistochemical analysis of androgen effects on androgen receptor expression in developing Leydig and Sertoli cells. Endocrinology 138:1259–1266. [DOI] [PubMed] [Google Scholar]
- 21. Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, et al. (2004) Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proceedings of the National Academy of Sciences of the United States of America 101:6876–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verhoeven G, Willems A, Denolet E, Swinnen JV, De Gendt K (2010) Androgens and spermatogenesis: lessons from transgenic mouse models. Philos Trans R Soc Lond B Biol Sci 365:1537–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arambepola NK, Bunick D, Cooke PS (1998) Thyroid hormone effects on androgen receptor messenger RNA expression in rat Sertoli and peritubular cells. J Endocrinol 156:43–50. [DOI] [PubMed] [Google Scholar]
- 24. Mayeda A, Krainer AR (1992) Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell 68:365–375. [DOI] [PubMed] [Google Scholar]
- 25. Hanamura A, Caceres JF, Mayeda A, Franza BR Jr, Krainer AR (1998) Regulated tissue-specific expression of antagonistic pre-mRNA splicing factors. Rna 4:430–444. [PMC free article] [PubMed] [Google Scholar]
- 26. Caceres JF, Stamm S, Helfman DM, Krainer AR (1994) Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science 265:1706–1709. [DOI] [PubMed] [Google Scholar]
- 27. Han SP, Tang YH, Smith R (2010) Functional diversity of the hnRNPs: past, present and perspectives. Biochem J 430:379–392. [DOI] [PubMed] [Google Scholar]
- 28. Hastings ML, Wilson CM, Munroe SH (2001) A purine-rich intronic element enhances alternative splicing of thyroid hormone receptor mRNA. Rna 7:859–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jannini EA, Crescenzi A, Rucci N, Screponi E, Carosa E, et al. (2000) Ontogenetic pattern of thyroid hormone receptor expression in the human testis. J Clin Endocrinol Metab 85:3453–3457. [DOI] [PubMed] [Google Scholar]
- 30. Romert P, Gauguin J (1973) The early development of the median thyroid gland of the mouse. A light-, electron-microscopic and histochemical study. Z Anat Entwicklungsgesch 139:319–336. [DOI] [PubMed] [Google Scholar]
- 31. Fagman H, Andersson L, Nilsson M (2006) The developing mouse thyroid: embryonic vessel contacts and parenchymal growth pattern during specification, budding, migration, and lobulation. Dev Dyn 235:444–455. [DOI] [PubMed] [Google Scholar]
- 32. Walker WH, Cheng J (2005) FSH and testosterone signaling in Sertoli cells. Reproduction 130:15–28. [DOI] [PubMed] [Google Scholar]
- 33. Lamont KR, Tindall DJ (2010) Androgen regulation of gene expression. Adv Cancer Res 107:137–162. [DOI] [PubMed] [Google Scholar]
- 34. Joyce KL, Porcelli J, Cooke PS (1993) Neonatal goitrogen treatment increases adult testis size and sperm production in the mouse. J Androl 14:448–455. [PubMed] [Google Scholar]
- 35. Sharpe RM, McKinnell C, Kivlin C, Fisher JS (2003) Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125:769–784. [DOI] [PubMed] [Google Scholar]
- 36. Nuclear Receptors Nomenclature Committee (1999) A unified nomenclature system for the nuclear receptor superfamily. Cell 97:161–163. [DOI] [PubMed] [Google Scholar]
- 37. Rao JN, Liang JY, Chakraborti P, Feng P (2003) Effect of thyroid hormone on the development and gene expression of hormone receptors in rat testes in vivo. J Endocrinol Invest 26:435–443. [DOI] [PubMed] [Google Scholar]
- 38. Ortega FJ, Moreno-Navarrete JM, Ribas V, Esteve E, Rodriguez-Hermosa JI, et al. (2009) Subcutaneous fat shows higher thyroid hormone receptor-alpha1 gene expression than omental fat. Obesity (Silver Spring) 17:2134–2141. [DOI] [PubMed] [Google Scholar]
- 39. Timmer DC, Bakker O, Wiersinga WM (2003) Triiodothyronine affects the alternative splicing of thyroid hormone receptor alpha mRNA. J Endocrinol 179:217–225. [DOI] [PubMed] [Google Scholar]
- 40. Thijssen-Timmer DC, Schiphorst MP, Kwakkel J, Emter R, Kralli A, et al. (2006) PGC-1alpha regulates the isoform mRNA ratio of the alternatively spliced thyroid hormone receptor alpha transcript. J Mol Endocrinol 37:251–257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression and localization of THRA in mouse testes. Sections of adult mouse testes were immunostained with antibodies to THRA (A) and the Sertoli cell marker GATA4 (B). Insets show higher magnification. Yellow indicates colocalization of THRA and GATA4 (C). Bars = 50 µm.
(TIF)
Expression and localization of HNRNPA1, SRSF1, and HNRNPH1 in mouse testes. Sections of adult mouse testes were double-immunostained with antibodies to HNRNPA1 (A), SRSF1 (D), HNRNPH (G) and the Sertoli cell marker GATA4 (middle panels, B, E, H). Insets show higher magnification. Yellow indicates colocalization of HNRNPA1, SRSF1, or HNRNPH and GATA4 (C, F, I). Bars = 50 µm.
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.